touch screen standard model patient monitor free sample
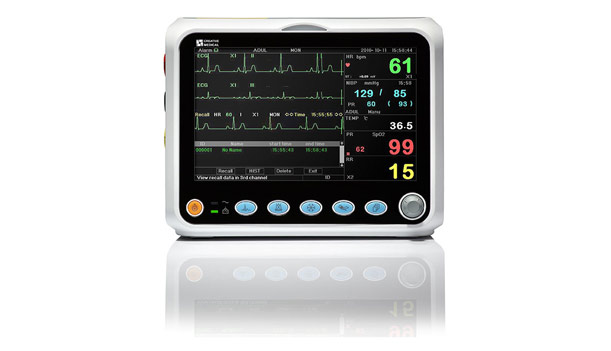
Soma Tech Intl offers the Axia V1500A Touch Screen monitors up to 50% below OEM prices with the same service and warranty as new. Soma technically and cosmetically refurbishes these patient monitors back to the original engineering manufacturer specifications. Using highly skilled, trained, and certified in-house biomedical engineers these units are fully tested, disassembled, and if necessary, parts are replaced. Once all the parts of the Axia V1500A Touch Screen Monitor are in working order, the patient monitor is calibrated back to OEM specifications, to make sure it works exactly the way it did when it originally left the manufacturer. Soma Tech Intl not only refurbished the product, but each unit also goes through a Special cosmetic restoration process. The Axia V1500A Touch Screen is cleaned and minor scratches and dentures are repaired, and decals are replaced if necessary.
Soma Tech Intl also purchases used and pre-owned patient Monitor. If you or your facility wants to sell your Axia V1500A Touch Screen, Soma Tech Intl has an experienced purchasing department that makes the process headache and hassle-free. When selling your patient monitor to Soma, there is no need for a middle man, it is a direct transaction to Soma which ensures that you get top dollar for your medical equipment. Soma Tech Intl not only buys patient monitors but a wide variety of different medical equipment as well if your medical facility is looking to free up space. If you have medical equipment you would like to sell to soma, send us a message here!
Soma Tech Intl offers a wide range of patient monitors. If you have any questions about any of our monitors or need a quick quote, call 1-800-GET-SOMA and one of our knowledgeable sales representatives will help you.

Multi-parameter patient monitors support the conduct of patient care in doctors’ offices, outpatient facilities, hospital operating rooms, hospital critical care facilities, and during EMS and non-emergency ambulance transport. There may also be a need for bedside measurement of vital signs in low-acuity post-anesthetic care and during sleep studies. Patient monitors are used to monitor adults, pediatrics and neonates.
Multi-parameter patient monitors provide the composite view required, at a glance. Many include configurable vital signs settings and both audio and visual comprehensive alarms. Various sizes exist, with variable numbers of parameters monitored. Portability and multi-use capabilities offer high cost-performance ratios by negating the need for multiple types of monitors. This vital equipment provides care teams more of the information they need while at the patient’s side.
Patient monitors are sometimes referred to as vital signs monitors, ECG monitors, EKG monitors or anesthesia monitors. Regardless of the label used, the multi-parameter nature and portability make the nearly dozen monitors offered by Rehabmart useful in a variety of settings.
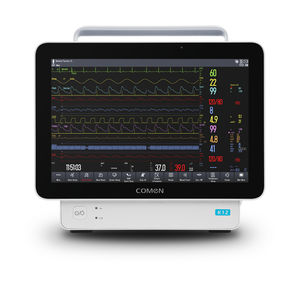
... associated with patient wellbeing revolves around being able to make the right decision at the right time, the Smartsigns Compact series of patient monitors rises to these challenges ...
The demands and dynamics associated with patient wellbeing revolves around being able to make the right decision at the right time, the Smartsigns Compact series of patient monitors rises ...
... (CMS) lets you centrally monitor the vital signs of up to 64 patients connected to Vista 120/Vista 120 S bedside monitors. This central surveillance streamlines workflow for clinicians, ...
... system features an intuitive Hospital Grade, IP54 rated touch screen and the ability to set up EEG display and trend analysis in addition to event management over a network. Continuously monitor ...
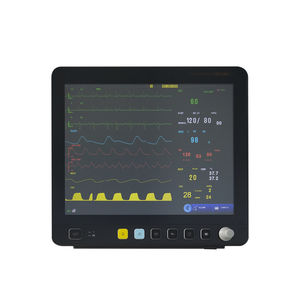
12.1 inch high resolution LCD display; 35 million clicking times long life touch screen with Accutouch technology; Maximum 168 hours graphic and tabular trends of all parameters; Adjustable ...
15 inch high-resolution LED backlight LCD with touchscreen;Modular design for convenient and efficient functions extension;High anti-interference ability with 8000V ECG isolation voltage ...
As we conducted this study at the University Hospital Zurich, all participants indicated to possess experience with patient monitors of the manufacturer Dräger (Drägerwerk AG & Co. KGaA. Lübeck, Germany), which were in use at the University Hospital Zurich at the time of the study. Additionally, about one of two participants had previous experience with monitors of Philips Healthcare (Koninklijke Philips N. V, Amsterdam, The Netherlands), and about one in six had previous experience with monitors of GE (General Electric Company, Boston, MA, USA).
Twenty-four (20%) participants addressed weight and industrial design issues of the monitoring devices, e.g., bulkiness. Ten (8%) participants complained about the technical characteristics of the displays. They wished for monitoring displays with these properties: “removable,” “large format,” “touchscreen,” “high-resolution”, “vibrant.” Nine participants (8%) mentioned problems with individual components of the monitors. Mainly issues with battery life and built quality of parts, e.g., connectors.5.
The anesthesiologists complained about insufficient standardization in monitoring - in both, the hospital and the global healthcare context. Unfavorably mounted monitors, poor operating room lighting conditions, the requirement for a large number of individual monitors, e.g. respirator, patient monitor, syringe pumps, to capture the situation were all mentioned as problematic. A few participants mentioned the poor transport capability of monitors as bothersome.
Twenty-six participants (21%) provided ideas that did not fit into any of the major topics outlined above. These included aspects relevant for safety design, e.g., the wish of an anesthesiologist that trend images should always be visible and slow changes over time should be made recognizable for the care provider, or that the alarm off button should only mute the alarm for which it is pressed. Alarm tones should be made more explicit. One participant mentioned that despite all monitoring, we should never forget to look at the patient and the monitors: “Treat the patient, not the monitor.”
Yes, Hope Industrial touch screens use resistive technology, which is pressure-sensitive and can be used with any type of stylus, as long as it is not sharp or rough (which could damage the touch screen surface). Please contact our sales department for more information.
After initial setup, the touch screen should not require periodic re-calibration. Installing new drivers could erase a previous calibration and at time re-calibration is done by preference since some users prefer a different calibration style (e.g.: pointer centered on the finger-tip vs. centered on the finger).
Yes. Our touch screen drivers allow multiple displays to be connected to a single PC whether in mirroring mode (multiple screens showing the same desktop) or extended desktop (a single desktop stretched across multiple displays).
Our Windows drivers allow each touch screen to be calibrated independently whether you are using USB or Serial for connection to the PC. Once configured, the cursor will follow your finger to any connected touch screen. For configuration help or more information, please contact our support group for assistance.
Support for Linux-based operating systems is available through both native drivers, and by using driver-less methods that rely on the HID device compatibility of our touch screens. A full review of the available methods is available on our blog.
A search was conducted in EMBASE and Ovid MEDLINE. Citations were screened for relevance against predefined selection criteria based on the PICOTS (Population, Intervention, Comparator, Outcomes, Timeframe, and Study Design)
• Any wearable device worn or placed on a body part to record a particular physiological change (e.g., respiratory rate sensors or blood pressure monitors).
Specific exclusion criteria were applied to narrow the focus of selected studies to those reporting patient health data captured via noninvasive RPM digital technologies. The predefined exclusion criteria included the following: interventions with invasive or implantable digital technology (e.g., implantable cardiac defibrillators, blood glucose monitors) or nondigital technology (i.e., landline telephone as the only source of data transmission); no remote monitoring of patient data (e.g., treatment algorithm); no real-time data capture (i.e., participants can only access the device at prescheduled times); and if the data acquired by a device were limited to only patient-reported outcomes, survey responses, or drug performance/adherence.
All citations identified from the literature search were screened for relevance. The first level of screening involved an assessment of citation abstracts for relevance by a single reviewer, based on specific exclusion criteria. Two reviewers were consulted to determine whether any uncertain abstracts were to be included. The second level of screening involved a review of the full-text articles identified from the level one screening to determine if these studies met the predefined inclusion and exclusion criteria listed above. This was undertaken by a single reviewer with four additional reviewers consulted to determine whether any uncertain articles were to be included or excluded based on the study selection criteria.
Shane-McWhorter, 2014United StatesMetabolic disorders/Type 2 diabetes, hypertensionObservational/Average duration 7 months10940–64Multiple components/One of two telemonitoring delivery methods: touch-screen or an IVR system both included BP monitor heart rate monitoring and patients were provided a digital scalePositivePharmacist, Healthcare educatorsGovernment
Michelle A. Adams, BSJ, MA, of Write All, Inc., Sonoma, CA, Tara Cowling, MA, MSc, and Brittany Gerber, MA, of Medlior Health Outcomes Research Ltd. provided medical writing and editorial assistance for this article. Brooke Rakai, PhD for providing research assistance in screening, data extraction, and quality appraisal and Krista White, MA for quality appraisal. Rosemary Besrutschko, MLIS Manager, Novartis Knowledge Center Information Delivery, was the medical librarian who assisted in the EMBASE and OVID search.




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey