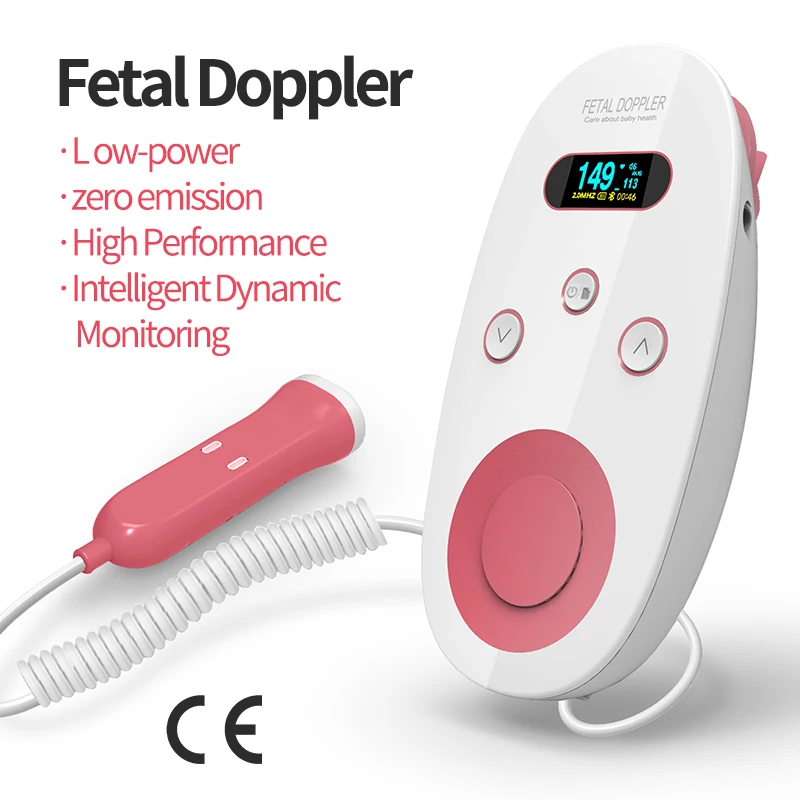
ce certification lcd panel factory

What are the criteria and procedure for CE marking of LCDs and which institutions can I contact? A liquid crystal display is a kind of flat panel display. It is used for the screen display of televisions and computers. The advantages of this display are low power consumption, small size, and low radiation. The LCD uses a liquid crystal solution in two polarized materials, so the current flowing through the liquid causes the crystals to rearrange themselves to achieve the purpose of the image. If you need to apply for CE certification, you can contact our company BEST ONE Inspection for the procedure.
CE marking provides a single technical specification for products from all countries for trade in the European market and simplifies trade procedures. In order to enter the European Union and the European Free Trade Area, products from all countries must be CE certified and have the CE mark on the product. CE marking is therefore the passport for products to enter the EU and EFTA markets.
CE certification means that the product meets the safety requirements set out in the EU Directive; it is a company’s commitment to consumers and increases their confidence in the product; products bearing the CE mark reduce the risk of being sold on the European market.
CE consists of EMC (Electromagnetic Compatibility) + LVD (Low Voltage Directive), EMC also includes EMI (interference) + EMC (anti-interference), LVD is also commonly known as SAFETY safety, general low voltage products AC less than 50V, DC less than 75V can not LVD project. Low voltage products only need to test EMC and issue CE-EMC certificate, high voltage products need to test EMC and LVD and issue two certificates and reports CE-EMCCE-LVD.
Safety design documents (including main design drawings, i.e. design drawings indicating creepage distance, clearance distance, number and thickness of insulation layers).
the certificate of registration of the product in the EU (for certain products, such as Class I medical devices, general IVD in vitro diagnostic devices).
EU laws, regulations, and harmonized standards are not only numerous but also very complex, so seeking the help of an EU notified body is a wise move to save time and effort and reduce risks.

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

A: All of our TFT LCDs meet or exceed 130 degree (Horizontal) and 110 degree (Vertical) viewing angle. Our wide view film technology achieve viewing angle 160 degree (Horizontal) and 140 degree(Vertical). We also have Super-Wide-View technology that will achieve 85/85/85/85 viewing angle, which is the best technology for viewing angle and color reproduction. Please consult the individual part specification for details.
A: NCM enables vivid reproduction of even the most subtle colors, such as pastels and flesh tones, and provides independent control of six colors without one color influencing any of the others. It supports 18-bit and 24-bit color and full motion video and compensates for any color shifts caused by LCD components such as CCFLs and optical films. Read the NCM Technology WhitePaper.
A: There are basically two approches to increase a display"s sunlight readability: increase the brightness level of the display, or reduce the ambient light reflection on the display surface. Good Display has several technology for outdoor readable applications, such as high brightness panel, transflective panel, anti-reflection and antiglare surface treatment, for more information, please check Outdoor Use TFT Displays for more information.
A: Yes. Good Display TFT LCDs response time is between 16ms to 25ms. Most of our newly developed LCD panels have 16ms response time, which is enough for moving picture. Please check the individual display specification for detail information.
A: LCD displays typically feature a wider viewing angle from straight on or above, compared to the viewing angle from below. The reverse-scan feature enables customers to select either a normal scan or reverse scan to obtain the best intended image, depending on whether the mounted display will be viewed typically from above or below. Good Display TFT LCDs have reverse scan function.
A: The defination of the CCFL lifetime is when the brightness level droped to 50% of its initial full brightness level. All the CCFL backlights for TFT LCDs are rated at 50,000 hours minimum. But most of the CCFL lamp unit can be replaced easily.
A: For industrial grade LED backlits TFT-LCD, the minimum lifetime is 60K hours. Be sure to consult the individual part specification for information on a specific LCM.
A: Many of the Good Display TFT LCDs operate at both 3.3 and 5V. However, as the 3.3V becomes the new standard, the LSI manufactory might decide to only support 3.3V, that is not controlled by Good Display. Please check the individual part specification for information.
A: The Mean time between failures (MTBF) data is not generally available. Under special circumstances MTBF data can be provided, but this is the exception, not the norm. Please contact your representative for further information.

CE Certification is a mandatory marking that indicates conformity in accordance with the legal and technical directives of the European Union (EU). CE has been in effect since 1993 and is not necessarily a marking of quality but the CE Certification is known as a ‘passport’ for EU-wide free flow for a wide range of industrial products.
The CE Mark certifies that a variety of products have met the relevant environmental, health and safety requirements of the European Union, and therefore ensures safety for consumers and the workplace.
In order for manufacturers to market their products in the EU and abroad, products must meet the relevant CE mark requirements. Once the CE mark has been approved, the products can be marketed within the EU without having to undertake further tests and modifications for each country.
Underwriters Laboratories does not necessarily approve products as opposed to evaluating materials, systems, components and products for the relevant compliance to the UL requirements. In order to carry a UL certification mark, the products must remain compliant with the standards set by UL.
UL and ULC ensures that companies supply safer products to the US, Canadian and global markets. The certification also improves the safety of products, as well as increases consumer confidence in their compliance and is regarded as a trusted symbol of safety.
RoHS (Restriction of Hazardous Substances) is an EU Directive that relates to electrical and electronic equipment placed on the European market. The RoHS Directive is closely linked with WEEE Care (the Waste Electrical and Electronic Equipment Directive), which controls the collection and recycling of electrical and electronic equipment in order to solve the problem of toxic waste.
RoHS aims at reducing hazardous substances being utilised in electrical and electronic equipment, while WEEE Care aims at reducing electrical and electronic equipment in products entering waste.
RoHS certification states that from 1 July 2006, electrical and electronic equipment that is put on the market cannot exceed maximum values of any of the following six substances.
The RoHS Directive applies to those who manufacture Electrical and Electronic Equipment (EEE), those who import these goods into the EU; those who export to other Member States; and those who re-brand equipment produced by others.
The primary aim of WEEE Care is to control the Waste of Electrical and Electronic Equipment and to recover and re-cycle such waste in order to reduce the volumes of electrical and electronic waste being disposed to landfill. WEEE Care arranges the collections and recovery of equipment to recycle in an environmentally friendly way. WEEE Care is a European environmental directive that aims to reduce the impact of disposing of electrical and electronic equipment in the environment.
The RoHS directive bans certain levels of substances being used in electrical and electronic equipment and WEEE Care aims at the correct disposal of this equipment.

We have recently upgraded our product certification. We have conducted CE,FCC and ROHS certification for the latest certification standards for our Interactive LCD Digital Display and All in one kiosk and Stretched displays. The certification details as following:

Our products are widely recognized and trusted by users and can meet continuously changing economic and social needs for Interactive Led Wall,3d Advertising Display,P3.91 Indoor Led Display,Perimeter Led Display Screen,Led Wall.Our final goal is "To try the best, To be the Best". Please feel free to contact with us if you have any requirements. The product will supply to all over the world, such as Europe, America, Australia, Hyderabad ,Turkmenistan ,Vancouver ,Really should any of these items be of interest to you, please let us know. We will be pleased to give you a quotation upon receipt of one"s detailed specifications. We"ve our personal specialist R&D enginners to meet any of the requriements, We look forward to receiving your enquires soon and hope to have the chance to work together with you inside the future. Welcome to take a look at our organization.

A Machinery shall be CE Marked before it is placed on the market or put into service or use. Normally a Machinery has an Electrical Equipment made of one or more control panels and components “in the field”.
The Control panel is often engineered and manufactured by a different producer then the machinery itself and it falls under the Low Voltage Directive 2014/35/EU (if powered above 50Vac).
That was interpreted in the following way: Manufacturer P of the control Panel sells to Manufacturer M of the Machinery and is not obliged to CE Mark its product.
In other terms: manufacturer “P1” of a part of the control panel sells it to the final manufacturer “P2”, who assembles the product and sells it to “M”. P2 has to CE mark the product before it is transferred to “M”.
As for “making available”, the concept of placing on the market refers to each individual product, not to a type of product, and whether it was manufactured as an individual unit or in series.
Not all experts think that way.There is a school of thought that strasses the fact that there is no specific product standard for Industrial Control panels in Europe: therefore the product cannot be CE Marked. The only way to support that reasoning is to demostrate the ICP is not a product falling under the Low Voltage Directive, in other termsit is not an Electrical Equipment. Here the definition taken from the Guide to the LVD:
Electrical Equipment: item used for such purposes as generation, conversion, transmission, distribution or utilisation of electrical energy, such as machines, transformers, switchgear and controlgear, measuring instruments, protective devices, wiring material, current-using equipment.
Nobody argues a swichgear must be CE Market - it actually has a product standard: EN 61439-1. Than, what is the difference between a Distribution Panel and an Industrial Control Panel? An ICP is not an Electrical Equipment, falling under the LVD, because it does not have a specific product standard? We consider that reasoning "weak".

Let iCertifi help you with your CE approvals. Since we are a consulting firm, we offer a different approach to homologations. Since we are not a test laboratory, we have partnerships with several test laboratories worldwide. We will negotiate the pricing from multiple test labs to ensure you obtain the best pricing possible. Since we are not tied to a single laboratory, we can also find laboratories with the best completion time. This allows you to obtain your certifications much faster which will allow your time to market to be expedited.
We have helped many companies become certified to sell their products in the European Union. Our experience in accordance with CE norms and our international partnerships with laboratories and regulators allows you to engage a single company for all your International Market Access (IMA) needs.
Product manufacturers needed to display the CE mark(stamp) on their product. The process of getting a CE mark(stamp) may seem vague. An outline of the process is as follows:
Distributors of products requiring CE marking should be inspected to ensure that this brand is present and properly positioned in the items. Distributors must also examine the accompanying documentation to ensure that the CE mark is obtained legally and correctly.
If you are a merchant for a product that is distributed in the European Economic Area, please contact us for professional certification services. We can help you to ensure that your products are on a legal level of safety, a level of health and environmental protection.
Postal stamps are established in accordance with the EC directives. This Directive is a legal act of the European Union (EU). The EU has established product codes for many industries in 1985. These standards are standards of health, safety and environmental protection.
Presently, set standards and legal standards are managed by two separate entities. While the European Union has established compliance with CE rules, you do not have to go through the EU. Instead, compliance can be applied in different ways.
1. Each distribution company has the opportunity to check the compliance efforts of their manufacturers. If the distributor checks their client’production, the manufacturer of the product must provide evidence of their CE mark.
iCertifi can assist you with your CE certification which often is the first step in International Market Access. If your ambitions are to sell your products globally, iCertifi can assist you with this process as well. We are proficient in certifications such as; CE, FCC, Industry Canada (IC), safety type approval (CB Scheme, IRAM S-Mark), Energy Efficiency such as CONUEE in Mexico and more. iCertifi can also provide you with local applicant services in any country you wish to penetrate. Let us help you eliminate the burdens to International Market Access. Contact us at (541) 275-5020 to get started on your CE certification.
CE Certificate of Compliance allows products to move freely and sell on the European market. This brand offers consumers proof that the product meets the environment, health and safety standard set by the EEA. Performance requirements are not a guarantee of quality. This simply means that the product fulfills the conditions for sale in the EU.
It is important to note that the CE marking does not mean that the product is made in the EEA. The European conformity marking indicates that it has reviewed, approved and pass the environment, safety and health that the EEA has established for a product to be sold in the EEA.

We pursue the management tenet of "Quality is superior, Service is supreme, Reputation is first", and will sincerely create and share success with all clients for Lcd Bar, Small Lcd Monitor, Touch Screen Computer, Lcd Monitor,Customize Ips Lcd Module. The concept of our corporation is "Sincerity, Speed, Services, and Satisfaction". We"re going to follow this concept and gain more and far more customers" pleasure. The product will supply to all over the world, such as Europe, America, Australia,Boston, Washington,Amman, United States.Besides there are also professional production and management , advanced production equipment to assure our quality and delivery time , our company pursues the principle of good faith, high-quality and high-efficiency. We guarantee that our company will try our best to reduce customer purchase cost, shorten the period of purchase, stable products quality, increase customers" satisfaction and achieve win-win situation .

Our corporation puts emphasis about the administration, the introduction of talented staff, plus the construction of team building, attempting hard to improve the quality and liability consciousness of team members. Our organization successfully attained IS9001 Certification and European CE Certification of Tft Lcd Touch Screen Display, Graphic Lcd Module, Interactive Display, Industrial Touch Screen Monitor,Transparent Lcd Screen. We warmly welcome all standpoint inquiries from home and abroad to cooperate with us, and anticipate your correspondence. The product will supply to all over the world, such as Europe, America, Australia,India, French,Ukraine, El Salvador.We sincerely hope to cooperate with customers all over the world, if you would like to have more information, please kindly contact us, we are looking forward to building up a great business relationship with you.

The CE mark is a mandatory conformity mark on many products sold in the single market European Economic Area (EEA). The CE mark certifies that a product has met EU consumer safety, health, or environmental requirements.
By affixing the CE marking to a product, a manufacturer declares, on his sole responsibility, that the product meets all the legal requirements for the CE marking, which means that the product can be sold throughout the European Economic Area (EEA, the 27 Member States of the EU and European Free Trade Association (EFTA) countries Iceland, Norway, Liechtenstein). This also applies to products made in other countries which are sold in the EEA.
The CE mark is a declaration by Hope Industrial that the product conforms with applicable requirements set out in EU law necessary before applying the mark. In order for an industrial monitor or related product to display the CE mark, Hope Industrial products have met some or all of the following Directives:EC-DirectiveSubjectDocument No.Low Voltage DirectiveProducts with a voltage rating of 50-1000 VAC or 75-1500 VDC2014/35/EU
Electromagnetic Compatibility (EMC) DirectiveApparatus liable to cause electromagnetic disturbance or which is liable to be affected by such disturbance2014/30/EU
More information on CE marking requirements is available directly from the European Commission, including "The Blue Guide," which provides detailed information.
RoHS Directive 2011/65/EU (Restriction of Hazardous Substances) is an included requirement for displaying a CE mark and restricts the use of some hazardous substances in electronics and electrical equipment sold in Europe. The Directive bans selling new products that contain more than specified levels of the following materials in the EU market: lead, cadmium, mercury, hexavalent chromium, polybrominated biphenyl (PBB) and polybrominated diphenyl ether (PBDE) flame retardants.
RoHS Directive 2015/863/EU amends the directive above and adds four additional substances, Bis(2-Ethylhexyl) phthalate (DEHP), Benzyl butyl phthalate (BBP), Dibutyl phthalate (DBP), Diisobutyl phthalate (DIBP).
RoHS-compliant parts must have less than or equal a maximum concentration of 0.1% (by weight) of lead, hexavalent chromium, mercury, PBB, PBDE, DEHP, BBP, DBP, DIBP and 0.01% for cadmium, unless the parts qualify for an exemption.

With each passing year, shipping domestic products overseas has become more confusing. Currently, the European Union requires that any product sold there must pass certain requirements before it can cross the border. These New Approach Directives, written by the European Commission and enacted through national legislation, are mandatory for all member countries.
The directives aim to simplify and regulate the movement of goods into and within the EU. In order for the EU to monitor these goods, they require manufacturers to display a CE Marking on applicable products or packaging, and on any accompanying literature. In areas where a directive is in force, it is a crime to place a product on the market without this CE Marking-the manufacturer is legally responsible for ensuring that the product complies with the directive and for placing the CE Marking on the product. The manufacturer also will be held legally responsible for any wrongful placement of the CE Marking on a product.
Any directive that applies to the EU and its products is required for any country that sells products there. Thus, as each new directive comes into effect, CE Marking for U.S. goods in that category becomes mandatory. U.S. manufacturers must become aware of the directives and their objectives. The directives are in place to ensure the production of safe devices that are fit for their designated purpose, promote manufacturer responsibility, maintain efficient regulation, benefit the community and control market orientation.
Many U.S. manufacturers are finding that they must place the CE Marking on their products to conduct business abroad. The EU is a critical market because it makes up the bulk of the European Economic Area. Established in 1994, the EEA is the largest economic region in the world-consisting of Austria, Belgium, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Liechtenstein, Luxembourg, The Netherlands, Norway, Portugal, Spain, Sweden, and the United Kingdom. While not EU members, Iceland, Norway and Liechtenstein are in the European Free Trade Association, which has different rules regarding directives.
CE Marking is not a quality standard; it is simply a manufacturer"s declaration that a product complies with the essential requirements of the relevant directives. Because CE Marking merely indicates that the product conforms to the minimum legal requirements, it does not ensure that the goods are quality products. It simply allows for the movement of goods and the removal of nonconforming products within the EU.
CE Marking is not intended as a marketing tool. In the United States, however, customers often look for the CE Marking on a product to see that it meets these minimum requirements, which other domestic products may lack.
The Medical Device Directive is currently receiving the most attention. This article therefore uses it as an example to show the steps of CE Marking. The MDD protects patients and operators through the safety, health and quality requirements of medical devices. Its 23 articles explain how it works, with carefully defined steps to guide manufacturers through the conformity procedures. Mandatory in June 1998, the MDD has prompted medical device makers to initiate efforts to affix CE Marking to their products before they lose their eligibility to ship to the EU.
The MDD contains four medical device classes-I, IIA, IIB and III. For Class I devices, manufacturers must perform a self-declaration, but no notified body is involved. Class I device manufacturers are on their own, except if the device includes a measurement or sterility function, which requires a notified body to come in to assess the device. For classes IIA, IIB and III, a notified body must be involved.
Obtaining CE Marking can be a long and tedious process, but with the help of a notified body, it need not be overwhelming. Yet sooner or later, companies must undergo the process to continue exporting to the large EU market.
For example, the MDD contains five steps: determine classification, meet essential requirements, set up the technical documentation, choose the conformity assessment procedure route and write the declaration of conformity. After completing these steps, manufacturers may affix CE Marking to their medical devices.
Before beginning the process, however, manufacturers must first obtain a copy of the directive. (Directives can be found at Web site europa.eu.int/eur-lex/en/search.html. Input the directive number in the search field.) Reading through the document will help them understand what the process entails and what it will mean for selling their product. After examining the 23 articles, the manufacturer should gain a precursory understanding of the process.
After understanding the process to follow, the manufacturer begins the classification step. To put the directive into perspective, the MDD is divided into annexes, which are like the chapters of a book and help the manufacturer classify the device. The MDD"s 12 annexes are:
In order to determine which stipulations the device must meet and whether it must be tested, the manufacturer starts with Annex IX. This section categorizes the product through a series of criteria. The manufacturer must classify the device or device family based on the classification ruling spelled out in Annex IX. In other words, the manufacturer must read the entire annex and decide which specific rules apply to the product.
The next step deals with meeting essential requirements, which Annex I outlines. The manufacturer must read the specifics under Annex I and determine which points apply to the device. Some of these specific classification requirements include device categories such as: chemical, physical and biological; infection and microbial contamination; constructional and environmental; measuring functions; and devices with energy sources.
Before moving on, the manufacturer must decide which "harmonized standards" fit with the product. The directives require that whenever possible or appropriate, the manufacturers follow harmonized standards. Harmonized standards can be European norms, British standards or whatnot, but they must be accepted by the EU and published in the Official Journal of the European Communities(also at Web site europa.eu.int/comm/dg03/directs/dg3b/newapproa/eurstd/harmstds). Manufacturers must identify all harmonized standards that apply to the type of directive they are using-each directive includes a list of those it requires.
In other words, the harmonized standards are compiled in a list of norms that have been approved and accepted by all EU members, and the manufacturer is responsible for identifying those standards that apply to them and showing evidence of compliance to those harmonized standards. This information is then compiled in the technical file, the next step in the CE Marking process.
A technical file is essentially the evidence that the manufacturer"s device meets Annex I requirements. Information in a technical file shows compliance to Annex I Essential Requirements. The manufacturer can determine how to set up its technical file; the only stipulation is that the documentation be available to Competent Authorities (EU members) for review when needed. The notified body simply reviews the technical file, verifies that it has the correct elements and observes that it has a control system.
Following the right pathNext, the manufacturer must choose a conformity assessment procedure route. If the manufacturer says that the device meets a certain criteria, it must prove that it has performed that type of testing. Along the same lines, if a section of the directive doesn"t apply to the product, the manufacturer must explain why it does not apply.After determining the criteria to meet, the manufacturer hires a notified body to perform the product assessments. This third-party certification body is a systems registrar that can assess and provide quality certification and serve as a testing agency. Notified bodies must be authorized to perform assessments of certain devices based on their technical expertise. They must also be nominated by an EU member government and notified by the European Commission.
The notified body evaluates the system against harmonized standards, such as ISO 9000 and EN 46000, to assure that a quality system is in place and all essential requirements are met. The notified body certifies that the product meets requirements by verifying conformity and, if applicable, testing the manufacturer"s devices. Again, the designation is based on expertise-a notified body is only designated to conduct a conformity assessment and is restricted to audit and ensure that the product meets the directive"s requirements for those areas in which they are certified. Certain notified bodies are not authorized to conduct assessments of certain devices because they do not have the technical expertise on their staff. Also, notified bodies may provide information or helpful tools, but not consulting services.
The notified body can guide the manufacturer through the difficulties of the CE Marking process while performing the necessary tasks to complete the process, including performing the assessment to the manufacturer"s selected annex route, ensuring the device complies to that annex, notifying the manufacturer that the device has passed successfully and issuing a CE certification. The manufacturer can then begin affixing the CE Marking to the product as well as its packaging and manuals.Further considerationsBecause the MDD allows for self-declaration for Class I devices, some companies have wrongfully affixed the CE Marking to their device without going through the proper procedures and channels. Either those companies did not understand the directive for self-declaration or they knew that notified bodies were not involved in the process, and without proper guidance, they took the chance that they would not be caught. This only applies to a small group of manufacturers with Class I devices for the MDD, but it nonetheless poses a problem for the EU.Other considerations are language requirements for the countries into which the manufacturer is selling, as well as vigilance systems and complaint handling. Each EU country has its own requirements for handling these processes.
Keep in mind that the MDD and others are new directives, with many gray areas. Test cases will determine in which direction they will head, but there are still many conflicting interpretation issues and confusion within the European countries surrounding the directives. Becoming familiar with directive requirements and following all procedures will help alleviate any misunderstandings and keep the process flowing to its conclusion-CE Marking.About the authorKorina Ortiz is business development manager for BSI Inc., the North American division of BSI, the world"s leading registrar of quality management systems. For more information, please contact BSI Inc. at (800) 862-4977.
If you take a close look at many of the objects you use each day—keyboards, monitors, headphones, and more—you may notice they have a symbol on them that looks like this:
This is the CE marking, and it signifies that the product you’re holding has been assessed and certified to meet safety, health, and environmental requirements in the European Economic Area (EEA). The EEA is simply the countries that make up the European Union (EU), plus a few additional European countries that are not formally part of the EU.
Even if you don’t live within the EEA, you’ll still find the CE marking on many of the products you purchase, as those products require the marking in order to be marketed in the EEA.
For our purposes, the most important point to keep in mind is that every medical device needs a CE marking in order to be sold in the EEA, even devices with the lowest-risk.
Before you begin the process of obtaining that CE marking, there are three questions commonly asked by medical device manufacturers that you should know the answers to if you want to fast-track your medical device onto the EU market:
Everyone wants to know that the products they use every day are safe and effective. And for certain product groups, like medical devices, there is too much riding on their performance to leave anything to chance. That’s why the need for a CE marking and the process for obtaining it exist.
CE marking stands for “Conformité Européene” (which translates to European Conformity) and it serves as proof that the product has been assessed by its manufacturer and found to meet all applicable EU requirements.
Keep in mind, the CE marking is considered the manufacturer’s declaration that their product meets all applicable legal requirements. However, while the manufacturer is technically responsible for declaring their device’s conformity, this doesn’t mean the European Commission isn’t enforcing its own regulations.
Most medical device manufacturers will need to have their quality management system (QMS) audited by an independent notified body (NB) before they can obtain a CE marking for their device. A notified body is a private entity that a member state of the EU has given authority to assess the conformity of medical devices with all applicable regulatory requirements.
Once a manufacturer has passed their certification audit, declared conformity, and affixed their CE marking, consumers should have confidence that the product meets high health, safety, and environmental standards.
You must classify and assess your medical device. To do so, you’ll need to consult Annex VIII of the EU Medical Device Regulation (EU MDR). There, you’ll find a rule-based classification system that will help you identify which of the four risk classes (or subclasses) your device falls under. The classes are as follows:
Next you shouldestablish a QMS for your device. The MDR is aligned (though still awaiting official harmonization) withISO 13485:2016, the international standard for medical device quality management systems, so following ISO 13485:2016 is the best way to meet the QMS requirements for compliance with MDR.
After implementing a QMS, you’ll need toproduce a technical filethat provides details on the conformity of your device and shows that you are satisfying requirements as set forth in the MDR.
However, if you have a class III device, you’ll also need to produce a Design Dossier, which includes in-depth administrative and technical information that helps facilitate the design examination performed by your notified body.
However, Class IIa, Class IIb, and Class III devices must all undergo a certification audit by a notified body. Additionally, the three subclasses of Class I devices—Is, Im, and Ir—must also use a notified body.
Once you have self-certified or fully passed the certification audit by a notified body, your final step is todeclare the conformity of your medical deviceand affix the CE marking to your product.
The MDR is clear: medical device manufacturers must have a QMS in place and it must include certain minimum requirements, which are outlined in Article 10 of the MDR, ‘General obligations of manufacturers.’
Fortunately, ISO 13485:2016 covers all of these requirements. And while the MDR does not force manufacturers to use ISO 13485, this standard is the only QMS standard that has been added to the list of standards proposed for harmonization in the EU.
With so much riding on obtaining your CE marking, it simply doesn’t make sense to rely on legacy QMS tools that leave you vulnerable when your notified body shows up for an audit.
Greenlight Guru offers the world’s only Medical Device Success Platform (MDSP) exclusively for medical devices with the latest regulatory best practices built into every feature. We work tirelessly to ensure our platform is always up-to-date with the latest standards and regulations so that you can bring safe, effective products to market faster—and keep them there longer.
Looking for a design control solution to help you bring safer medical devices to market faster with less risk? Click here to take a quick tour of Greenlight Guru"s Medical Device QMS software

We consistently carry out our spirit of ""Innovation bringing development, Highly-quality ensuring subsistence, Management promoting benefit, Credit attracting customers for CE Certification High-end Slide Cash Drawer,CE Certification Pos Display Mounting Solutions,CE Certification Pos Machine,OEM Touchscreen Signage,OEM All In One Touch Kiosk.We sincerely welcome you come to visit us. Hope we have good cooperation in the future. The product will supply to all over the world, such as Europe, America, Australia, Nepal ,Ecuador ,Estonia ,Please feel cost-free to send us your specifications and we"ll respond to you asap. We"ve got a professional engineering team to serve for the every single detailed needs. Free samples may be sent for you personally to know far more facts. So that you can meet your desires, please really feel cost-free to contact us. You could send us emails and call us straight. Additionally, we welcome visits to our factory from all over the world for much better recognizing of our corporation. nd merchandise. In our trade with merchants of several countries, we often adhere to the principle of equality and mutual advantage. It is our hope to market, by joint efforts, both trade and friendship to our mutual benefit. We look forward to getting your inquiries.

On basis of professional talents and abundant fund, several agents have been set up in many cities, providing fast and thorough service. Secondly, during the guarantee period, we will send new lights with new order for small quantity. For defective batch products, we will repair them and resend them to you or we can discuss the solution including re-call according to real situation.




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey