ce certification lcd panel free sample

CE Certification is required for all recreational boats entering or being sold in the European Union. Manufacturers must test and document to ensure conformity to all applicable European directives and requirements. CE certification is obtained from Notified Bodies, organizations that are recognized by European states to conduct CE assessments and issue CE certification documents.
NMMA works closely with the International Marine Certification Institute (IMCI), a notified body in Europe that issues conformity certificates, to assist U.S. boat builders in the certification process. Certification by a notified body enables you to display the CE mark on your products and allows you free and open access to the European Union market.

The CE mark is a mandatory conformity mark on many products sold in the single market European Economic Area (EEA). The CE mark certifies that a product has met EU consumer safety, health, or environmental requirements.
By affixing the CE marking to a product, a manufacturer declares, on his sole responsibility, that the product meets all the legal requirements for the CE marking, which means that the product can be sold throughout the European Economic Area (EEA, the 27 Member States of the EU and European Free Trade Association (EFTA) countries Iceland, Norway, Liechtenstein). This also applies to products made in other countries which are sold in the EEA.
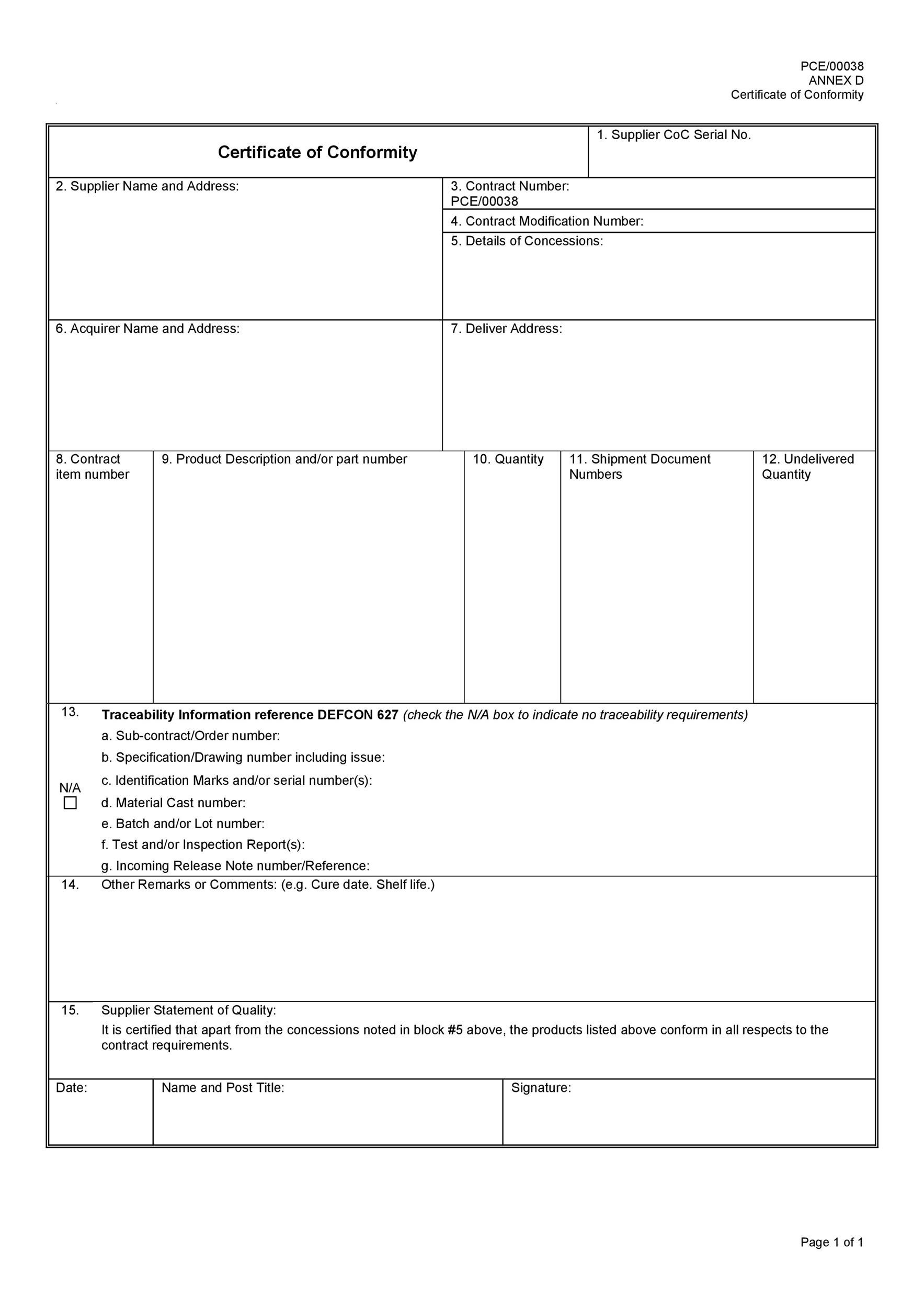
The CE mark is a declaration by Hope Industrial that the product conforms with applicable requirements set out in EU law necessary before applying the mark. In order for an industrial monitor or related product to display the CE mark, Hope Industrial products have met some or all of the following Directives:EC-DirectiveSubjectDocument No.Low Voltage DirectiveProducts with a voltage rating of 50-1000 VAC or 75-1500 VDC2014/35/EU
Electromagnetic Compatibility (EMC) DirectiveApparatus liable to cause electromagnetic disturbance or which is liable to be affected by such disturbance2014/30/EU
More information on CE marking requirements is available directly from the European Commission, including "The Blue Guide," which provides detailed information.
RoHS Directive 2011/65/EU (Restriction of Hazardous Substances) is an included requirement for displaying a CE mark and restricts the use of some hazardous substances in electronics and electrical equipment sold in Europe. The Directive bans selling new products that contain more than specified levels of the following materials in the EU market: lead, cadmium, mercury, hexavalent chromium, polybrominated biphenyl (PBB) and polybrominated diphenyl ether (PBDE) flame retardants.
RoHS Directive 2015/863/EU amends the directive above and adds four additional substances, Bis(2-Ethylhexyl) phthalate (DEHP), Benzyl butyl phthalate (BBP), Dibutyl phthalate (DBP), Diisobutyl phthalate (DIBP).
RoHS-compliant parts must have less than or equal a maximum concentration of 0.1% (by weight) of lead, hexavalent chromium, mercury, PBB, PBDE, DEHP, BBP, DBP, DIBP and 0.01% for cadmium, unless the parts qualify for an exemption.

A Machinery shall be CE Marked before it is placed on the market or put into service or use. Normally a Machinery has an Electrical Equipment made of one or more control panels and components “in the field”.
The Control panel is often engineered and manufactured by a different producer then the machinery itself and it falls under the Low Voltage Directive 2014/35/EU (if powered above 50Vac).
That was interpreted in the following way: Manufacturer P of the control Panel sells to Manufacturer M of the Machinery and is not obliged to CE Mark its product.
In other terms: manufacturer “P1” of a part of the control panel sells it to the final manufacturer “P2”, who assembles the product and sells it to “M”. P2 has to CE mark the product before it is transferred to “M”.
As for “making available”, the concept of placing on the market refers to each individual product, not to a type of product, and whether it was manufactured as an individual unit or in series.
Not all experts think that way.There is a school of thought that strasses the fact that there is no specific product standard for Industrial Control panels in Europe: therefore the product cannot be CE Marked. The only way to support that reasoning is to demostrate the ICP is not a product falling under the Low Voltage Directive, in other termsit is not an Electrical Equipment. Here the definition taken from the Guide to the LVD:
Electrical Equipment: item used for such purposes as generation, conversion, transmission, distribution or utilisation of electrical energy, such as machines, transformers, switchgear and controlgear, measuring instruments, protective devices, wiring material, current-using equipment.
Nobody argues a swichgear must be CE Market - it actually has a product standard: EN 61439-1. Than, what is the difference between a Distribution Panel and an Industrial Control Panel? An ICP is not an Electrical Equipment, falling under the LVD, because it does not have a specific product standard? We consider that reasoning "weak".

The CE mark indicates that a product is compliant with all applicable directives and regulations – which in turn requires the CE mark. Such ‘CE marking directives’ and regulations apply to a wide range of products, including electronics, toys, helmets, sunglasses, and medical devices.
In this guide, we list the directives and regulations for which the CE mark is required. Each directive and regulation also includes a product list andother requirements that EU importers and manufacturers must be aware of.
The European Commission requires that manufacturers place the CE mark on their products if such products are covered by one or more of the CE marking directives or regulations. Additional requirements also apply, as we explain in the next section.
Currently, there are more than 20 CE marking directives and regulations. Each one covers a certain product scope and describes the technical and regulatory requirements for manufacturers, importers, and distributors.
Many CE marking directives and regulations do not list the specific products under their scope. Instead, these list the general product scope (e.g. input and output voltage). Therefore, it is sometimes confusing for manufacturers or importers to determine which particular directive or regulation applies to their products.
CE marking directives and regulations specify technical, regulatory, environmental, or other requirements for manufacturers, importers, or distributors. Each directive and regulation have different requirements for the product it covers. Here we summarized some general requirements:
The Radio Equipment Directive (RED) establishes a regulatory framework, including electrical safety, electromagnetic compatibility, radio spectrum use efficiency, and other circulation requirements, for the radio equipment placed in the EU market.
Note that radio equipment is defined as any electrical or electronic device that intentionally emits and/or receives aiming at radio communication and/or determination.
Also, according to the directive, if the manufacturer or importer demonstrates compliance via relevant harmonized standards, then the conformity assessment procedure might be completed without Notified Body involvement.
However, if the manufacturer or importer has not applied harmonized standards – or such standards do not exist for the product – then a Notified Body shall be required, either via EU-type examination or conformity based on full quality assurance.
According to Article 2 of the Directive, ‘radio equipment’ is defined as any electrical or electronic product that intentionally emits and/or receives radio waves for the purpose of radio communication. This can include WiFi, LTE, 5G, Bluetooth or GPS-enabled devices.
a. EN 303 354 V1.1.1 Amplifiers and Active Antennas for TV Broadcast Reception in Domestic Premises; Harmonised Standard Covering the Essential Requirements
b. EN 300 422-1 V2.1.2 Wireless Microphones; Audio Pmse Up to 3 GHz; Part 1: Class a Receivers; Harmonised Standard Covering the Essential Requirements
The RoHS Directive restricts the use of hazardous substances in electrical and electronic equipment (EEE) and establishes waste disposal methods for such products in order to protect human health and avoid environmental pollution.
The RoHS Directive applies to almost all electronic products placed in the European Union market, although there are some exemptions listed on the annexes of the directive.
b. EN IEC 63000: Technical Documentation for the Assessment of Electrical and Electronic Products with Respect to the Restriction of Hazardous Substances
The Ecodesign Directive sets up the regulatory framework for improving the performance of the so-called “energy-related products”, that is electrical and electronic products that might have a big impact on energy consumption.
The directive mainly sets compulsory requirements on the energy efficiency of household appliances and other products, with the goal of protecting the environment.
The Toy Safety Directive establishes safety requirements for toys and certain types of children’s products intended to be used by children under 14 years of age.
It requires that products under the scope of the directive must follow the technical requirements regarding the chemicals and heavy metals concentration limit, mechanical/physical properties specification, flammability rate, and more.
Annex I of the directive lists exempted products such as puzzles with more than 500 pieces, and bicycles with a maximum saddle height higher than 435 mm.
The Personal Protective Equipment (PPE) Regulation establishes designing and manufacturing requirements for personal protective equipment (PPE) placed in the EU market for the purpose of protecting the health and safety of the user, either on worksites or other places that present potential physical danger.
The regulation classifies PPE into Category I, II, or III, depending on the level of risk of the environment associated with their use. The hazardous levels are arranged in ascending order.
Annex I of the Personal Protective Equipment Regulation established three risk classes for which PPE is intended to protect users. Below we explain the differences among these classes.
contact with cleaning materials of weak action or prolonged contact with water; contact with hot surfaces not exceeding 50 °C or other types of relatively minor risks take place in the working environment. Examples of Category I PPE include:
Protective equipment that is intended for military use, self-defense, and resistance to non-extreme climate conditions are often exempted from the PPE Regulation, although there are some exemptions, which you can find in the text of the regulation itself.
The Construction Products Regulation (CPR) established rules and standards for products used for construction purposes in the EU. The Regulation provides a regulatory framework to assess the performance of construction products from the perspective of mechanical resistance, stability, flammability, health, environmental impact, and more.
According to Annex III of the regulation, when the product is covered by harmonized standards then Notified Body involvement might not be necessary in order to complete a declaration of performance.
“‘construction product’ means any product or kit which is produced and placed on the market for incorporation in a permanent manner in construction works or parts thereof and the performance of which has an effect on the performance of the construction works with respect to the basic requirements for construction works;”
The Medical Devices Regulation applies to medical devices and their accessories and establishes a regulatory framework for the safety and health of the patients and users.
“This Regulation lays down rules concerning the placing on the market, making available on the market or putting into service of medical devices for human use and accessories for such devices in the Union. This Regulation also applies to clinical investigations concerning such medical devices and accessories conducted in the Union.”
‘Medical Device’ can mean any instrument, apparatus, appliance, material, or other articles intended to be used for diagnosing, preventing, monitoring, predicting or alleviating disease, disability, physiological or pathological problems.
The regulation classifies medical devices into four classes: Class I, IIa, IIb, and III, where the medical devices of Class III hold the highest risk. The higher the number is, the stricter the rules that apply to the products.
Class IIa: Medical devices that have some potential low to medium risk and can generally be used for less than 30 days. Products belonging to this category include surgical gloves, hearing aids, and diagnostic ultrasound machines.
Class IIb: Medical devices that might constitute medium to high-risk to the patients and are generally designed to be used for more than 30 days. Products belonging to this category include long-term corrective contact lenses and surgical lasers.
Class III: Medical devices that have the potential highest risk. Products such as cardiovascular catheters, aneurysm clips, hip-joint implants, and prosthetic heart valves are considered as Class III medical devices.
a. EN IEC 60601: Medical Electrical Equipment – Part 2-83: Particular Requirements for the Basic Safety and Essential Performance of Home Light Therapy Equipment
b. EN ISO 17664: Processing of Health Care Products – Information to Be Provided by the Medical Device Manufacturer for the Processing of Medical Devices – Part 1: Critical and Semi-critical Medical Devices
The In-Vitro Diagnostic Medical Devices Regulation lays down rules and regulatory requirements for in-vitro diagnostic medical devices intended to be used by humans in the EU.
c. EN ISO 25424: Sterilization of Health Care Products – Low Temperature Steam and Formaldehyde Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices
The Measuring Instruments Directive regulates measuring instruments placed in the market of the EU. In particular, according to the regulation, measuring instruments shall provide an accurate measurement.
This directive stipulates that, for all non-automatic weighing instruments, it is compulsory to conduct a product performance assessment and ensure the products meet all essential requirements of the EU legislation before being marketed among the member states of the EU. Notified Body involvement is generally required.
“This Directive, which comes under the SAVE programme concerning the promotion of energy efficiency in the Community, determines the efficiency requirements applicable to new hot-water boilers fired by liquid or gaseous fuels with a rated output of no less than 4 kW and no more than 400 kW, hereinafter called ‘boilers’.”
The Noise Emission in the Environment Directive regulates the noise emissions into the environment generated by machinery used in outdoor areas, including the conformity assessment procedure and technical documentation.
The directive provides two options for certification of the regulated outdoor machinery. When such equipment is subject to permissible sound power levels, the Notified Body involvement is required in the aspects of product design manufacturing procedures.
The Gas Appliances Regulation covers a wide range of household appliances function by means of burning gaseous fuels for the purpose of cooking, heating, refrigerating, lighting, and washing. The regulation establishes harmonized standards and procedures from the aspects of design, structure, and safety.
“ ‘appliances’ means appliances burning gaseous fuels used for cooking, refrigeration, air-conditioning, space heating, hot water production, lighting or washing, and also forced draught burners and heating bodies to be equipped with such burners.“
The Pressure Equipment Directive regulates pressure equipment and assemblies with a maximum allowable pressure greater than 0.5 bar gauge in terms of safety requirements, product design, and manufacturing procedures.
The directive classifies pressure equipment into four categories, ranging from I to IV. The hazardous levels are arranged in ascending order. Conformity with the directive requires Notified Body involvement. Note, however, for a product classified under the category I is generally optional.
“ ‘pressure equipment’ means vessels, piping, safety accessories, and pressure accessories, including, where applicable, elements attached to pressurised parts, such as flanges, nozzles, couplings, supports, lifting lugs.”
The Simple Pressure Vessels Directive establishes procedures for simple pressure vessels in terms of design, manufacturing, safety, conformity assessment procedures, free movement, and other essential requirements for these products.
The Lifts Directive regulates lifts in terms of design, manufacture, components, installation, safety rules, and maintenance methods. The directive provides standards for manufacturers, importers and owners of lifts to refer to.
(c) goods alone if the carrier is accessible, that is to say a person may enter it without difficulty, and fitted with controls situated inside the carrier or within reach of a person inside the carrier.”
The Cableway Installations Regulation sets up rules for cableways designed with the purpose of transporting people. This regulation involves requirements for the design, components, construction, subsystems, installations, infrastructure, operation, safety analysis, and the process of the cableway entry into service in the EU market.
a. EN 1709: Safety Requirements for Cableway Installations Designed to Carry Persons – Precommissioning Inspection, Maintenance, Operational Inspection and Checks
The Rail System Interoperability Directive sets standards for community rail systems concerning the design, construction, placement in service, upgrading, renewal, operation, and maintenance of the system, as well as the health condition of the staff who operate the system.
The ATEX Directive defines the essential health and safety requirements and conformity assessment procedures for equipment and protective systems used in an environment with potential explosive hazards in the EU market.
Most of the products regulated by this directive require Notified Body involvement. However, there are some exceptions, which can be found in Article 13 of the Directive
‘pyrotechnic article’ means any article containing explosive substances or an explosive mixture of substances designed to produce heat, light, sound, gas or smoke or a combination of such effects through self-sustained exothermic chemical reactions.”

Shenzhen YIPI Electronic Limited, established in 2 0 1 3, is a professional manufacture and exporter which focus on the research, development, production, sales and service of Laptop & Monitor Privacy Screen, Privacy filters and all kind of screen protect

By placing the CE marking on a product a manufacturer is declaring, on his sole responsibility, conformity with all of the legal requirements to achieve CE marking. The manufacturer is thus ensuring validity for that product to be sold throughout the EEA. This also applies to products made in third countries which are sold in the EEA and Turkey.
Not all products must bear the CE marking. Only those product categories subject to specific directives that provide for the CE marking are required to be CE marked.
CE marking does not mean that a product was made in the EEA, but states that the product is assessed before being placed on the market. It means the product satisfies the legislative requirements to be sold there. It means that the manufacturer has checked that the product complies with all relevant essential requirements, for example health and safety requirements.
If you are importing a product that is from a third country you have to check that the manufacturer outside the EU has undertaken the necessary steps. You must check that the documentation is available.
Even if your product is manufactured outside the EEA, you must ensure the product bears CE marking if your product comes under the scope of a directive requiring CE Marking. Not all products sold in the EU need to bear CE marking.
CE marking applies to products, ranging from electrical equipment to toys and from civil explosives to medical devices. The full list of these product categories is below:
If you have an enquiry about the Construction Products Regulations or if you would like information on the new requirements for structural steel, which came into force on 1 July you can email construction.products@communities.gov.uk.
Before you place a CE marking on a product, you need to establish which EU New Approach Directives apply to your product. You must not attach a CE marking to a product outside the scope of the directives.
There are more than 20 directives setting out the product categories requiring CE marking. The essential requirements that products have to fulfil, for example safety, are created at EU level and are set out in general terms in these directives. Harmonised European standards are issued with reference to the applied directives and express the essential safety requirements in detailed technical terms.
Each directive covering your product specifies whether an authorised third party (Notified Body) must be involved in the conformity assessment procedure necessary for CE marking. This is not obligatory for all products, so it is important to check whether the involvement of a Notified Body is required. These bodies are authorised by national authorities and officially ‘notified’ to the European Commission and listed on the NANDO (New Approach Notified and Designated Organisations) database.
If you manufacture a product it is your responsibility to test the product and check its conformity to the EU legislation (conformity assessment procedure). One part of the procedure is, as a general rule, a risk assessment. By applying the relevant harmonised European standards, you will be able to fulfil the essential legislative requirements of the directives.
The CE marking must be placed on the product by the manufacturer, or by his authorised representative within the EEA or Turkey. It must be placed according to its legal format to the product or its data plate. It must be visible, legible and impossible to remove. If a Notified Body was involved in the production control phase, its identification number must also be displayed. It is the manufacturer’s responsibility to draw up and sign an ‘EC DoC’ proving that the product meets the requirements. That’s it, your CE-marked product is ready for the market.
Once you have satisfied the conformity assessment requirements for CE marking you must attach the CE marking to your product or its packaging. There are specific rules for using the CE marking for your product, as well as rules for the reproduction of the CE marking logo.
In general you should attach the CE marking to the product itself but it may also be placed on the packaging, in manuals and on other supporting literature. Rules covering the use of the CE markings vary depending on the specific EU directive that applies to the product and it is advisable to study the applicable guidance. The following general rules all apply:
Member states will ensure they implement the regime governing the CE marking. They will take appropriate action in the event of improper use of the marking and provide for penalties for infringements, which may include criminal sanctions for serious infringements. Those penalties will be proportionate to the seriousness of the offence and constitute an effective deterrent against improper use
The general principles of the CE marking are contained within Regulation (EC) No 765/2008 which sets the requirements for accreditation and market surveillance relating to the marketing of products. You can read the CE marking regulations on the Europa website.
the CE marking is placed onto the product or to its data plate - if this is not possible or not warranted because of the nature of the product, it must be placed onto the packaging and accompanying documents
You must keep certain documentation once you have placed the CE marking onto your product. This information can be requested at any time by the Market Surveillance Authorities to check that a CE marking has been legitimately placed on a product.
There are many bodies that enforce CE marking legislation to prevent misuse of the CE marking and to ensure that product safety is maintained to a high standard.
Enforcement, or market surveillance, is undertaken by nominated public authorities (Market Surveillance Authorities) in each member state, and each state has separate ways of enforcing the legislation once it has been implemented into national law.
Market Surveillance Authorities and processes will vary depending on which directives are applicable to your product. The following bodies, amongst others, are responsible for CE marking enforcement in the UK:
If an enforcement body finds your product does not meet CE marking requirements, they will often provide you with an opportunity to ensure it is correctly CE marked. If you fail to comply with this, you will be obliged to take your product off the market. You may also be liable for a fine and imprisonment.

The European New Approach Directives form the starting point of the CE marking (Conformité Européenne). In these directives, user manuals are also discussed extensively. What do the European New Approach Directives mean and what do they mean for you and the user manuals of your products?
The directives give essential requirements regarding health, security, the environment, and consumer protection for products that are traded within the European Economic Area (EEA). The directives form the starting point of the CE marking. The above-named essential requirements apply to different products. For example, there are directives for machines and medical devices, but also for pressure vessels and toys.
How do these directives come into being? First, the European Commission, the Advisory Commission, or the business marshal will form a draft directive. Then the European Council of Ministers determines whether the directive is adopted or not. If the directive is adopted, member states are obliged to receive the requirements linked to the directive in their national legislation. This should happen within a certain period of time, which usually takes two years. The period until the admission of the directive is generally called the ‘transition period’. After that, the directive is binding and in principle, there can’t be made any heavier or more additional requirements by the member states. Often this results in existing laws that need to be changed to avoid contradictions or duplicates.
The importer of products from outside the EEA: s/he is entirely responsible for the placing of the CE marking, even though that s/he is not authorized by the manufacturer.
The responsibility for the CE marking lies only at the trading company when it is regarded as a manufacturer or importer. This is the case if the trading company:assembles the product itself;
sells the product under his own name (private label). The new seller of course must apply the required data on the product, needs to draw their own EC declaration of conformity and needs to change the personal data in the user manual. The initial concept is the data of the supplier;
The manufacturer needs to perform several actions in order to meet the requirements for the CE marking. These various actions are clearly listed below:
Even if a trading company is not regarded as a manufacturer, it still has certain responsibilities in the field of CE marking. The action plan below puts these in a row:
The essential requirements are generally related to the technical safety aspects of the product itself. These are the ‘in practice’ requirements which you are most concerned with. You can find these requirements in Annex 1 of every New Approach Directive.
Government Inspectors will first check if the products meet these fundamental requirements during inspections. The fundamental requirements also include requirements regarding documentation and marking (user manual, installation instructions, maintenance prescriptions, EC declaration, CE marking). These requirements form the starting point for European standards. For example, the directives require adequate instructions for use. The European harmonized standard EN 82079–1 gives minimal requirements for instructions for use.
In most cases, the manufacturer or trader can certificate themselves. This ‘self-certification’ is usually also referred to as internal manufacturing inspection. If it regards products with an increased risk, an inspection agency (notified body) must be engaged.
If you assemble the product yourself, then it is very likely that you buy a large number of parts. In some cases, the supplier has to provide the parts with an affixed CE marking. But even if that is not the case, the parts must still comply with the CE requirements set by you, so that the final product can meet the essential CE requirements. For this reason, it is recommended to register the agreements about the CE marking, including the task division, in a repurchase agreement. Examples of what you can register in such an agreement:That the manufacturer puts together the Technical (Construction) Dossier and stores it.
Many products should include a manual to explain how to use the product safely. Mostly, the manual includes instructions for laypeople and service-maintenance instructions for specialized users. For consumer products, only a user manual is enough.
All potential dangers that come with the use of the product should be described in the manual sufficiently. Also, the possible dangers caused by improper use should be taken into account. Only when the manufacturer pays attention to the requirements, (professional) knowledge, and the experience of the user, warnings can be properly formulated. A manual should therefore always be written for that user.
The Technical (Construction) File defines the product design and the conditions for safe usage. The file contains information such as drawings, certificates, test reports, calculations, the user manual, part lists, etc. The Technical File is kept by the manufacturer, which keeps it available for competent national authorities. The physical presence of the dossier is not always necessary, but it must be available within a few weeks. In principle, the documents must be retained until 10 years after the manufacturing date of the product or after the last unit was produced (when the product is made in series). In some directives, the Technical File forms a key feature in the conformity assessment.
For each product or series of the same product that falls under a European New Approach Directive, an EC Declaration of Conformity should be prepared. This declaration indicates that the product complies with the requirements of the directive(s). When it comes to various directives, compliance can be represented within one EC declaration. It is however necessary to mention the data of the notified body that performed the EC type-examination or the quality system evaluation. The declaration is signed by an authorized person of the company, who uses the same language as used in the user manual. The EC Declaration of Conformity must, in some cases, be sent together with the product. In the Technical (Construction) Dossier a copy is enclosed. In other cases, it is sufficient to preserve the declaration yourself, depending on the directive that applies to the product.
The CE mark is only applied when the product meets all relevant European New Approach Directives. This is usually done on the identification plate, on a label, or with a sticker. Some directives allow that the CE mark is not applied on the product itself but on the packaging.

Available to Professional and Concierge account members only, your CE Compliance Transcript is the quickest way to know what your requirements are, what courses have been reported for you, and what you still need to complete.
Some professions have requirements that change with each renewal; for example, you may be required to take Recognizing Impairment every other renewal and Domestic Violence every third. Your personalized Transcript keeps track of this information so you"ll never have to wonder which requirements apply to you.
Once all of your requirements have been fulfilled, your Transcript will display 100% Complete and you can enjoy the peace of mind that you will be able to renew your license with ease. If it displays Not Complete, simply click Show me what I"m missing to be directed to your requirements. You will be able to see exactly how many hours you have left to complete, including any specific subject areas.

to your certificates; plus print, share, and download wherever and whenever you like. Each digital certificate has its own unique ID and QR code, making it easy to confirm that your employee’s certificate is valid and authentic.
The American Red Cross digital certificate is a first of its kind, online certificate that gives you anytime, anywhere access to your certification and training history.
Digital certificates can be viewed, printed or shared online and can be accessed anytime through your Red Cross Account. Each certificate includes a unique ID and a QR code which meets employment requirements and allows employers to easily confirm your certificate is valid. There is no need to carry your printed certificate around anymore!
Class participants and employers can visit redcross.org/take-a-class/digital-certificate and enter the ID found on the digital certificate (or scan the QR code with a standard QR reader using a smart device) to access a copy of the valid certificate with student training information.
Go to redcross.org/take-a-class/digital-certificate to search by email, name or cert ID. Check the box next to the certificate(s), click on “view and print” to display the certificate and “print” to obtain a hard copy.
Go to redcross.org/take-a-class/digital-certificate to search by email, name or cert ID. Check the box next to the certificate(s), click on add to cart. A $7.95 shipping and handling fee is charged for each printed certificate request, your request will be processed within three (3) business days. Certificates will be printed and mailed to the address provided via the US Postal Service, please allow up to ten (10) business days for certificate delivery. There is also an expedited shipping option that can be selected prior to adding the wallet card to your cart.
In certain cases, certification of skills is a job requirement. For example, CPR/AED and First Aid certifications are required if you are an EMT or a designated emergency responder at your work. In other cases, Red Cross certification is a way of demonstrating that you are prepared for a wide range of emergencies – in your personal life and in activities such as coaching, youth organizations, etc.
Professionals who need continuing education units to maintain licensure or certification can obtain CEUs through American Red Cross Training Services.
Once training has been successfully completed, a CEU certificate, along with a course certificate, will be sent to students who provided an email address. Students, who took training through a third-party provider, should contact the provider or instructor to obtain a copy of the CEU certificate. Class participants should verify with their appropriate accrediting organization that CEUs received from taking Red Cross courses will be accepted
The American Red Cross is approved to award CEUs through the Commission on Accreditation for Pre-hospital Continuing Education (CAPCE). The purpose of CAPCE is to standardize the review and approval of quality EMS Continuing Education activities.
The American Red Cross has been approved to provide continuing education credit through CAPCE for the following educational activities as of October 18, 2019.:

This is one of the most common UL Marks. If a product carries this Mark, Underwriters Laboratories found that samples of this product met UL"s safety requirements. These requirements are primarily based on UL"s own published Standards for Safety. This type of Mark is seen commonly on appliances and computer equipment, furnaces and heaters, fuses, electrical panelboards, smoke and carbon monoxide detectors, fire extinguishers and sprinkler systems, personal flotation devices like life jackets and life preservers, bullet resistant glass, and thousands of other products.
The European Commission describes the CE mark as a "passport" that allows manufacturers to circulate industrial products freely within the internal market of the EU. The CE mark certifies that the products have met EU health, safety and environmental requirements that ensure consumer and workplace safety. All manufacturers in the EU and abroad must affix the CE mark to those products covered by the "New Approach" directives in order to market their products in Europe. Once a product receives the CE mark, it can be marketed throughout the EU without undergoing further product modification.
Most products covered by New Approach Directives can be self-certified by the manufacturer and do not require the intervention of an EU-authorized independent testing/certifying company (notified body). To self-certify, the manufacturer must assess the conformity of the products to the applicable directives and standards. While the use of EU harmonized standards is voluntary in theory, in practice the use of European standards is the best way to meet the requirements of the CE mark directives. This is because the standards offer specific guidelines and tests to meet safety requirements, while the directives, general in nature, do not.
The manufacturer may affix the CE mark to their product following the preparation of a declaration of conformity, the certificate which shows the product conforms to the applicable requirements. They must maintain a technical file to prove conformity. The manufacturer or their authorized representative must be able to provide this certificate together with the technical file at any time, if requested by the appropriate member state authorities.
(5) The declaration must show the signature of a company official for purposes of the company assuming liability for the safety of its product in the European market. This European standards organization has set up the Electromagnetic Compatibility Directive. According to CE, The Directive basically states that products must not emit unwanted electromagnetic pollution (interference). Because there is a certain amount of electromagnetic pollution in the environment, the Directive also states that products must be immune to a reasonable amount of interference. The Directive itself gives no guidelines on the required level of emissions or immunity that is left to the standards that are used to demonstrate compliance with the Directive.
Like all other directives, this is a new-approach directive, which means that only the main requirements (essential requirements) are required. The EMC-directive mentions two ways of showing compliance to the main requirements:
Like all CE-related directives, this is a new-approach directive, which means that only the main requirements (essential requirements) are required. The LVD-directive describes how to show compliance to the main requirements.
The Canadian Standards Association (CSA) is a nonprofit association serving business, industry, government and consumers in Canada and the global marketplace. Among many other activities, CSA develops standards that enhance public safety.

It has long been thought that the quickest path to market for medical device manufacturers is to access the European market by obtaining a CE marking instead of going through the US FDA.
Although the compliance requirements are similar in many ways, the European route is thought to be less bureaucratic, resulting in shorter times to market, greater acceptance rates of new devices, and lesser costs associated with obtaining conformity certification.
While the benefits of obtaining a CE marking are significant, regulations are always subject to change. New modifications to the European medical device requirements are making the process more similar to that of the FDA when it comes to establishing conformity.
By May 2020, a new Medical Device Regulation (MDR 2017/745) will go into effect throughout the European Union. Despite the changes, there still remains a clear path to establishing conformity and obtaining a CE marking that will allow your company to access European markets.
This article describes the path to compliance under European regulation and provides five key steps medical device companies must take in order to obtain a CE marking that allows product distribution into the EU marketplace.
You"ll need to determine which set of regulations applies to your device based on the nature of the device itself. You"ll either be subject to the Medical Device Regulation (MDR 1017/745) or the In Vitro Diagnostics Regulation (IVD 2017/746).
European regulations require that you consult Annex VIII of the Medical Device Regulation (MDR) to classify your medical device according to its risk profile. Classification criteria are based on the duration of contact with the patient, the degree of invasiveness of the device, and the parts of the body affected by use of the device, among other items.
A provided decision tree uses 22 descriptive rules to help you classify your device as either Class I, IIa, IIb, or Class III, according to the associated risks. Once this is completed, you"ll understand exactly which regulations apply to your device.
Establishing a compliant quality management system is essentially a global requirement when it comes to entering the medical device market. The QMS standards set forth by the European Commission are similar to those of the FDA and other organizations, and many medical device companies use ISO 13485 as a guideline for building an effective quality management system.
Quality management systems are sets of policies and procedures that involve and affect everyone at your medical device company. This includes everyone from the document controller charged with oversight of your QMS to the workers that follow those policies and procedures daily to ensure that your design and manufacturing processes are safe and effective.
An eQMS designed for the medical device industry is the best means of ensuring that your efforts to guarantee product quality and effectiveness get the best results. You"ll benefit from cloud-based document storage, document version control, enhanced data security, and superior organization that reduces your time to market.
Now that you"ve properly classified your device and implemented a quality management system at your organization, the next step is to ensure that your device meets the conformity requirements set out in European Commission regulations.
For most medical devices, you"ll have to review the requirements of the Medical Device Regulation (MDR 2017/745), ensuring that your device is deemed acceptable across several dimensions, including its appropriateness for the intended use, safety, labeling and packaging, effects of transportation and storage, and risk versus benefit for the end user. Annex I of this document sets out the general safety and performance requirements for affixing the CE marking.
If your medical device is in risk classes I, IIa, or IIb, you"ll be required to produce a technical file on your medical device that provides details on the conformity of the device and shows that you satisfy the Essential Requirements.
The requisite information for creating such a document should already be contained in your QMS and should be easy to source and assemble, provided your organization has implemented a software-based, searchable QMS.
For Class III devices, a Design Dossier should be compiled which contains the data of the technical file along with a description of the design process for the device.
Now that you"ve identified and classified your device, implemented a QMS, and prepared the documentation to illustrate your conformity with EU regulations, you"ll need to have your QMS and documentation audited by a notified body. One thing to note is some Class I devices can be self-certified and do not require an audit by a notified body.
A notified body is a third-party company that has been accredited by European competent authorities to conduct audits of medical device companies, and their products and systems. There are several large, international auditing and standards organizations that provide this service.
Once you"ve successfully passed your audit, you should be issued a CE marking certificate for your product along with an ISO 13485 certificate that establishes that your QMS is compliant with European standards.
The final step is to create a Declaration of Conformity. This is a legally binding document which declares that your device meets all of the essential requirements as laid out by EU MDR and any other applicable regulatory standards.
Overall, the actual process for obtaining a CE marking defined in MDR 1017/745 is similar to the previous process from MDD. However, there are some higher level changes to be aware of, such as:
Inclusion of New Devices - The MDR covers some categories of medical devices that are not currently intended for medical use, but that carries some of the same risks as medical devices that are covered under the new regulations. This change has seen items like contact lenses, dental filling material, and even breast implants subject to medical device safety requirements.
Focus on Post-Market Surveillance - The new MDR places more stringent requirements on medical device companies in regard to post-market surveillance, with the expectation that companies will gather information about the safety and effectiveness of their device in practice by conducting regular clinical evaluations.
New Traceability Requirements - Manufacturers are required to affix device and product numbers to the items they sell, in order to facilitate enhanced traceability and unique identification of devices.
Looking for a design control solution to help you bring safer medical devices to market faster with less risk?Click here to take a quick tour of Greenlight Guru"s Medical Device QMS software →

Our target is to consolidate and improve the quality and service of existing products, meanwhile constantly develop new products to meet different customers" demands for Big Led Screen,Led Display Supplier,P4.81 Outdoor Led Display,Stadium Perimeter Led Display,Narrow Pixel Pitch Led.We warmly welcome friends from all walks of life to seek mutual cooperation and create a more brilliant and splendid tomorrow. The product will supply to all over the world, such as Europe, America, Australia, Croatia ,Bahamas ,Cape Town ,Please feel cost-free to send us your specifications and we"ll respond to you asap. We"ve got a professional engineering team to serve for the every single detailed needs. Free samples may be sent for you personally to know far more facts. So that you can meet your desires, please really feel cost-free to contact us. You could send us emails and call us straight. Additionally, we welcome visits to our factory from all over the world for much better recognizing of our corporation. nd merchandise. In our trade with merchants of several countries, we often adhere to the principle of equality and mutual advantage. It is our hope to market, by joint efforts, both trade and friendship to our mutual benefit. We look forward to getting your inquiries.
We consistently carry out our spirit of ""Innovation bringing development, Highly-quality ensuring subsistence, Management promoting benefit, Credit attracting customers for CE Certification High-end Slide Cash Drawer,CE Certification Pos Display Mounting Solutions,CE Certification Pos Machine,OEM Touchscreen Signage,OEM All In One Touch Kiosk.We sincerely welcome you come to visit us. Hope we have good cooperation in the future. The product will supply to all over the world, such as Europe, America, Australia, Nepal ,Ecuador ,Estonia ,Please feel cost-free to send us your specifications and we"ll respond to you asap. We"ve got a professional engineering team to serve for the every single detailed needs. Free samples may be sent for you personally to know far more facts. So that you can meet your desires, please really feel cost-free to contact us. You could send us emails and call us straight. Additionally, we welcome visits to our factory from all over the world for much better recognizing of our corporation. nd merchandise. In our trade with merchants of several countries, we often adhere to the principle of equality and mutual advantage. It is our hope to market, by joint efforts, both trade and friendship to our mutual benefit. We look forward to getting your inquiries.

There is a broad array of EU legislation pertaining to the marking, labeling, and packaging of products in the European Union. The first step in investigating the marking, labeling, and packaging legislation that might apply to a product entering the European Union is to draw a distinction between what is mandatory and what is voluntary. Decisions related to mandatory marking, labeling, or packaging requirements may sometimes be left up to individual Member States. Furthermore, voluntary marks and labels are used as marketing tools in some Member States but not in others. This section is focused primarily on the mandatory marks and labels seen most often on consumer products and packaging, which are typically related to public safety, health, or environmental concerns. It also includes a brief overview of a few mandatory packaging requirements, as well as more common voluntary marks or labels used in EU markets.
It is also important to distinguish between marks and labels. A mark is a symbol and/or pictogram that appears on a product or its respective packaging. These range in scope from signs of danger to indications of methods of proper recycling and disposal. The intention of such marks is to provide market surveillance authorities, importers, distributors, and end users with information concerning safety, health, energy efficiency and environmental issues relating to a product. Labels, on the other hand, appear in the form of written text or numerical statements, which may be required but are not necessarily universally recognizable. Labels typically indicate more specific information about a product, such as measurements or an indication of materials that may be found in the product (such as in textiles or batteries).
CE marking is probably the most widely used and recognized marking required by the European Union. Found in all “New Approach” legislation with a few exceptions, the placement of the CE mark on a product serves as the manufacturer’s declaration that the item meets all EU regulatory requirements (typically related to safety, health, energy efficiency, or environmental concerns) for marketing that item in the European Economic Area, which comprises the 27 Member States of the European Union and Iceland, Liechtenstein, and Norway. The CE mark does not certify that a European-based regulatory authority has approved a product for sale in the EU. CE marking is required for the following products and product families:
Not all products require a CE Mark. Only products that fall under the regulations or directives for the categories above have the CE Mark. EU law prohibits companies from attaching a CE mark on other products, such as cosmetics or chemicals. U.S. exporters should also note that companies often incorrectly or falsely CE mark their products.
Effective July 16, 2021, the EU required all CE marked products to have a label that identified a point of contact within the region. This requirement applies to products sold online and through traditional distribution channels. The label must give a contact name and address for customs and market surveillance authorities to reach out to for questions about the product. If an importer or distributor cannot fulfill that role, an exporter will have to appoint an Authorized Representative in the European Union or use a shipping platform to act in that capacity. The Authorized Representative is responsible for ensuring the availability of the conformity documentation, cooperating with market surveillance authorities, and informing authorities when they have reasons to believe that a product presents a risk. In March 2021, the European Commission published guidelines under Article 4 of the Regulation (Regulation (EU) 2019/1020)).
The e-mark is a mark for approved vehicles and vehicle components given by a national certifying authority. The certifying body issues an e-marking certificate after inspection and approval of compliance of a vehicle or its component. The number shown in the rectangle on the label indicates the Member State in which the approval process was conducted. A base approval number must also be provided adjacent to this certification. This four-digit number will correspond to the directive and type of device in question. The country-number correlation is as follows (this is not an exhaustive list):
The name, trade name and address, or registered office, of the manufacturer or person responsible for marketing the cosmetic product within the European Union.
Member States are to draw up procedures for providing the information set out above in the case of cosmetic products that have not been pre-packaged. The product function and list of ingredients must appear also on the container or packaging. Member States may stipulate that the information on the label is provided in their national or official language(s).
According to the Classification, Labelling and Packaging of Hazardous Substances (CLP) Regulation (Regulation 1272/2008), a label of dangerous substances must indicate the name of the substance; the origin of the substance, specifically, the name and address of the manufacturer or distributor; the danger symbol and an indication of danger involved in the use of the substance; and a reference to the special risks arising from such dangers.
The dimensions of the label must not be less than a standard A8 sheet of paper (52 mm x 74 mm), and each symbol must cover at least one-tenth of the label’s surface area. Member States may require their national language(s) to be used in the labeling of dangerous substances. If the packaging is too small, the labeling may be affixed in some other manner. The packaging of products considered dangerous, but are neither explosive nor toxic, may go unlabeled if the product contains such small quantities of dangerous substances that there is no danger to consumers.
Symbols must be employed if the substance can be defined as explosive, oxidizer, flammable, harmful, toxic irritant, corrosive, or harmful to environment. Containers of hazardous substances should include, in addition to the appropriate symbols, a raised triangle to alert the vision-impaired to their contents. Note that the CLP Regulation has undergone numerous amendments relating to, for example, the marking and labeling of additional substances, including implementing the classification, labeling, and packaging requirements for chemicals based on the United Nation’s Globally Harmonized System.
The WEEE directive (Directive 2012/19/EU) is designed to tackle the rapidly increasing waste of electrical and electronic equipment and it complements European Union measures on landfills and waste incineration. It also impacts the design of products in order to reduce material use and facilitate reuse and recycling. It sets collection, recycling, and recovery targets for all types of electrical and electronic equipment. Businesses should check requirements for the Member State to which the product will be imported. Depending on the country and quantities placed on the market, the party responsible for placing the product on the market will have to register with the appropriate authorities, join a producer compliance scheme, or set up an individual scheme to meet their take-back and recycling obligations. The wheel bin symbol indicates that the product is not to be discarded with normal household waste. In instances where this symbol cannot be displayed on the equipment itself, it should be included on the packaging.
Energy labels, according to Regulation 2017/1369, show how appliances rank on a scale from A (green), the most energy efficient, to G (red), according to their energy consumption. These labels apply to different categories of household appliances including air conditioners, refrigerators, televisions, washing machines, space heaters, and solid fuel boilers, among others. They are meant to help consumers choose the less energy consuming products and promote product ecologically friendly products. As of March 1, 2021, new energy labels include QR codes that consumers can scan. In order to facilitate the energy label use, the European Union maintains a site for generating energy labels.
In addition, since January 1, 2019, manufacturers, importers, and authorized representatives of non-EU manufacturers have to register all products requiring energy labels in the European Product Database for Energy Label and Eco-Design, access to which is available to EU consumers.
In addition to applying a CE marking for products falling under the ATEX Directive (2014/34/EC), which defines essential health and safety requirements and conformity assessment procedures of equipment or protective systems intended for use in potentially explosive atmospheres (e.g., offshore platforms, mines), it is necessary to display the “Ex” mark, which is a specific marking for hazardous location equipment showing compliance with the ATEX directive. A symbol designating the product group or category as specified in the directive will be located next to the “Ex” mark.
Regulation No 450/2009 on active and intelligent materials and articles or parts that come into contact with food includes labeling for the “Do Not Eat” symbol. To allow identification by the consumer of non-edible parts, active and intelligent materials and articles or parts, if they are perceived as edible, must be labeled with the words Do Not Eat and include, when technically possible, the above symbol.
The label must be legible, firmly secured, and accessible, and the manufacturer or the authorized representative established in the European Union is responsible for supplying the label and for the accuracy of the information contained therein. Only the information provided in the Directive needs to be supplied. No restrictions are preventing additional information from being included on the label.
The “wheel mark” shown above is the equivalent of CE marking for marine equipment. It applies to equipment for use on board any new ship in the European Union, wherever the ship is situated at the time of construction, and on equipment placed on board existing ships in the European Union, whether for the first time or to replace equipment already carried on board. It does not apply to equipment already on board on the date on which the directive entered into force in 1997. The directive applies to life-saving appliances, marine pollution prevention, fire protection, navigation equipment, and radio communication equipment. A revised Marine Equipment Directive (2014/90/EC) was adopted in July 2014 and is applicable since September 18, 2016.
Machines used outdoors are subject to CE marking requirements in line with the Outdoor Noise Directive (2000/14/EC). Along with the CE mark, products that fall under the Directive must also have marking, above, indicating the “guaranteed sound power level.”
The Mobius loop with a number at the center and a letter code indicates the type of plastic the packaging is made from. The above symbol is an example of how a plastic’s type may be indicated on a product. As part of the EU voluntary identification system for plastics, according to Decision 97/129/EC, the following marks are used for the most common types of plastics:
There are no EU-wide symbols used to designate the recyclable nature of glass. However, it is certainly encouraged on the national level with an array of symbols. The one shown above is a sample to show recyclability.
Textile products must be labeled or marked whenever they are put on the market for production or sale. The names, descriptions, and details of a textile’s fiber content must be indicated on products available to consumers. With the exception of trademarks or the name of the undertaking, other types of required information must be listed separately. Member States may require that their national language be used on the labeling. Marking required by the regulation (1007/2011/EU) include textile fiber names, related labelling, and marking of the fiber composition of textile products.
Tire label legislation (Directive 1222/2009/EC and Directive 228/2011/EC) requires that tire manufacturers declare fuel efficiency, wet pavement grip, and ext




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey