rhodium in lcd screen factory

On May 19, 2011, Deutsche Bank issued db Physical Rhodium ETC securities.Johnson Matthey recently (Nov. 15, 2011) forecast that the metal will remain in surplus (by 123,000 troy ounces (one troy ounce (oz) = 31.10 grams)) in 2011, and now its price has fallen from a "stratospheric" level of over $10,000/oz in June 2008 to "languish" around $1,700 (midprice on Nov. 30, 2011), somewhat lower than that of gold. So, what"s with rhodium?
The platinum group metals, or PGMs, of which rhodium is one, are a group of six metals clumped together pretty much in the middle of the periodic table. The others are iridium, osmium, palladium, platinum and ruthenium. The metal, which is extremely difficult to separate from the other metals with which it naturally occurs (including the other PGMs), is always produced as a byproduct of the extraction of these others; no such thing as a rhodium mine exists.
The English chemist, William Hyde Wollaston discovered the metal in 1803, soon after he discovered palladium and around the same time Smithson Tennant (also English) discovered both osmium and iridium. The rarity of the metal, the fact that it is a byproduct, and the complexity of (and costs involved in) its extraction have all, historically, contributed to robust pricing over the last 80 years, and especially in the last couple of decades.
An autocatalyst, which sits inside a motor vehicle"s catalytic converter (itself placed between its engine and muffler), is a metal, or ceramic, honeycomb coated with PGMs (of which rhodium is one) and various chemicals.
In gasoline-poweredvehicles, the autocatalyst converts over 90 percent of the carbon monoxide, oxides of nitrogen and unburned hydrocarbons into carbon dioxide, nitrogen and water vapor (often appearing as drips from out of the auto"s muffler). In diesel-powered vehicles, in addition to the equivalent amounts of hydrocarbons and carbon monoxide that are converted to more harmless compounds, so too is 30-40 percent of the potentially carcinogenic diesel particulate matter.
Since the first production vehicle was fitted with a catalytic converter back in 1974, their use has flourished and now catalytic converters are fitted to over 85 percent of all the new vehicles sold each year worldwide.
To put the effects they have in context, back in 1960, a gasoline-powered vehicle would typically, for every mile driven, spew out 100 grams of carbon monoxide, hydrocarbons and oxides of nitrogen. By 2004, this had been reduced to just some 2 grams, and autocatalyst development continues today.
Rhodium, because of its hardness and both its resistance to corrosion and high melting point (higher than that of platinum), is currently used in three main types of glass manufacturing, that of: thin-film transistor liquid crystal display (TFT-LCD) panels, glass fibers and, increasingly, in solar photovoltaic (PV) panels.
In the manufacture of TFT-LCD panels (used in TVs, monitors and displays), platinum and rhodium are used to line the channels, melting tanks and stirring cells, not only because they can withstand temperatures up to 1,650ºC, but also because they are inert. This last is of particular importance, as the glass substrate cannot contain any charge-bearing particles that may interfere with the function of the TFT laid down on it.
In the manufacture of glass fibers, the molten glass is drawn through an array of many tiny, uniform, orifices or nozzles, set in what is called a bushing — essentially just a box out of which they stick. These nozzles are made of a platinum/rhodium alloy.
Finally, rhodium is also used in the manufacture of the glass used in solar panels, which are required to be as defect free as possible and "highly transmissive."
In the chemical industry, rhodium catalysts are used in the production of aldehyde, which, with hydrogenation, leads to an oxo-alcohol, and in the production of acetic acid using the Monsanto process. (According to Johnson Matthey, the rising demand for rhodium in the chemical sector is being driven "by downstream demand for paints and adhesives, particularly in China.")
It will come as no surprise that by far the largest producer of rhodium is South Africa, which, in 2011, is forecast to produce some 650,000 oz out a total global supply figure for the mined metal of an estimated 768,000 oz. Recycling of autocatalysts is anticipated to amount to some 260,000 oz in 2011.
Source: Forecast production figures from Johnson Matthey, who notes that: "Supply figures represent estimates of sales by the mines of primary pgm and are allocated to where the initial mining took place rather than the location of refining."
Since primary rhodium is produced only alongside other PGMs, on the mining front, anyway, no rhodium mining "pure play" exists. And the big rhodium producers are, therefore, necessarily, the big producers of the other PGMs.
Investors can invest directly, buying the physical metal in ingot or as sponge, and "directly" through, e.g., Deutsche Bank"s Physical Rhodium ETC, this last giving the investor an entitlement to the physical metal.
As to the rationale behind an investment in rhodium, there a number of factors that should be carefully considered. Some of the more obvious are: Rhodium is, first and foremost, an industrial metal — with all that implies
There is also one other aspect of investing in rhodium (and some other industrial metals) that should be considered. While, according to Johnson Matthey, net inflows (to late September) to the Deutsche Bank ETC accounted only for about 14,000 oz, were such inflows to become significant, then any investment decision would need to factor in such demand, in addition to that from industry. This can only add further complexity to the investment process.

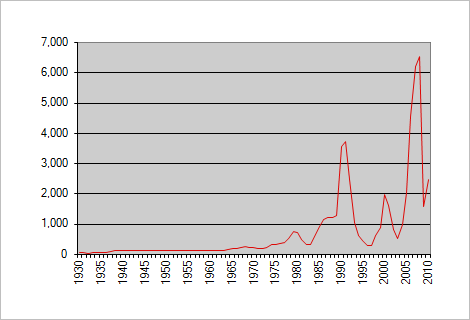
LONDON, Jan 10 (Reuters) - Prices of precious metal rhodium surged to a record high of $7,025 an ounce on Thursday as consumers in the glass-making and auto industries scrambled for scarce supplies, traders said.
Dealers said rhodiumwas quoted at $7,000/$7,050 an ounce, a gain of more than 25 percent since January last year and compared with the previous record high of $7,000 seen in 1980. On Wednesday it was quoted around $6,975/$7,025 an ounce.
Most rhodium is used by car makers in catalytic converters to limit carbon emissions, where regulations have become much stricter and contributed to rising demand for the metal.
Traders say that has been a major factor behind rhodium’s price rise over the last two years. Another is growing demand from glass makers ramping up production of flat panel screens used for televisions and computers.
South Africa is the world’s biggest producer of rhodium, which is a by-product of platinum. Supply disruptions in the country in recent months also have boosted rhodium prices.
During the manufacturing process, the molten glass is fed through a trough that is made out of the alloy, which can stand extreme heat and won’t melt.
Last December Corning announced capital expenditure between $1.5 billion to $1.7 billion to build additional capacity to meet growing demand for large flat-panel televisions.
“We expect that the LCD glass market will continue to grow into the next decade,” said James B. Flaws chief financial officer at Corning said on the company’s website.
Corning has previously said that it expects the overall LCD glass market to reach 1.7 billion square feet of glass in 2007 and to grow again by at least 400 million square feet in 2008.
Rhodium is an incredibly popular precious metal for many reasons. Its biggest draw is arguably the fact that it is trading at an extremely high price, currently over $18,000 per troy ounce, according to Johnson Matthey prices.
We hope this infographic helped you think of a couple places you might have rhodium scrap. If you do find any, be sure to sell it to a precious metals refiner like Manhattan Gold & Silver. We offer some of the quickest and fairest payments in the industry with a thorough assay process.

I used a different tool to separate the glass from the adhesive holding it to the aluminum on the back. I haven"t seen any of those screens your talking about. If you have some clear looking plastic that acts like a magnifying glass, it"s probably a fresnel lens. I"ve got a number here somewhere for a company that recycles and sells LCD screens. You can put some in with the circuit boards to be refined but they don"t want too many in there so they (SIPI) gave me a number but now I can"t find it, might have to call them back. So I put all the little LCD"s in with the boards like from phones games and calculators and save the large ones. That other pane of glass I just broke it up and threw it in with my other screens, I ain"t messing with it.

The word “rhodium” originates from the Greek word “rhodon,” which represents the lovely “rose.” When the metal was discovered, the scientist thought that the beautiful rose aptly describes its pinkish red color. Later on, however, the metal was found to have a shiny silver-white color in most cases.
Rhodium (Rh) belongs to the six member noble metals group that includes palladium, ruthenium, iridium, osmium and platinum. These metals exist in common rare metallic ores and exhibit some common characteristics. Rhodium is distinguished by its unique corrosion resistance, hardness, silvery-white metallic appearance and chemical inertness. It does not tarnish and is not prone to corrosion at normal room temperature. That is the secret of its durability, and it is one of the rarest precious metals.
After discovering the rare metal palladium, W. H. Wollaston discovered rhodium while he was trying to separate pure platinum from the ore. He used platinum ore from Peru as the raw material. After separating platinum and palladium from the ore sample, he was left with the residue of a red powder, which was later recognized as sodium rhodium chloride. Research studies state that he had used aqua regia, ammonium chloride and iron to separate palladium. By using hydrogen in the reduction process of the chloride salt of rhodium, he was able to separate rhodium, which had a pinkish hue. (Interested in discovering the properties of various materials? Be sure to read How to Get Started in a Career as a Materials Scientist.)
Rhodium is a highly valued precious material. Most of the metal is used as a catalyst (along with other catalysts) for automobile catalytic converters.
Rhodium compounds should be considered as toxic and carcinogenic. These can also cause strong stains on the skin. If the aerosol of this rare metal is inhaled it can be absorbed by the body, causing the risk of toxicity.
Because it is a rare mineral, there is insufficient data related to its safety. Hence, maximum precautions must be taken while handling, processing and using this material. (For more on safety, read The Dangers of Typical Corrosion Prevention Solutions.)
Rhodium displays many of the common properties of the rare platinum group metals (PGM), which generally have good chemical stability as well as catalytic properties. Additionally, rhodium is a good conductor of heat and electricity. It is corrosion resistant and stain resistant, and is one of the most reflective metals, which makes it a superior precious metal.
Low electrical contact resistance makes rhodium an ideal material for electrical contacts. It is durable, as it generally fails to oxidize even when heated. It absorbs oxygen while melting and releases the absorbed oxygen during solidification. It dissolves in aqua regia, but not in nitric acid.
It has a high melting point of 1,966°C (3,571°F). Thermocouples made of rhodium can accurately measure temperatures up to 1,800°C (3,272°F). This metal has a boiling point of 3,695°C (6,683°F). These properties ensure that it is suitable for high temperature applications.
Most of the reserves of the platinum group of metals (PGMs) are found in South Africa. Production of these metals involves refining the base metals as well as finishing refinery of the precious metals. Steps involved include floatation, comminution, smelting and final conversion. Chrome and oxide ore content often creates challenges for the PGM metal extraction. Proprietary processes using low temperature roasting and bromine (acid) leaching process are able to maximize the yieldof oxide ores and mixed ores containing rhodium (improved to the tune of 65% for rhodium and 85% for platinum).
The normal production process of rhodium is briefly described as follows:Noble metals such as platinum, gold and palladium are first separated by precipitation from the PGM ores
The addition of hydrochloric acid to rhodium hydroxide results in H3RhCl6 (a purified acid solution of rhodium), which is further added to sodium nitrite and ammonium chloride, enabling the precipitation of rhodium
The precipitate of rhodium is allowed to dissolve in hydrochloric acid and heated to remove contaminants by burning and thus the purified rhodium metal is finally produced
Most of the market demand for rhodium is driven by the demand for automobile catalytic converters in Japan, Europe and the United States, and the glass industry demand in Asia. As it is one of the rarest metals, the price is determined by the demand. According to market research studies, increasing Asian demand for the rare metal is due to producers of flat display glass panels.
Rhodium is sometimes applied as a decorative coating on jewelry made of silver and on circuit components, making these products free from tarnish and corrosion. It is applied on decorative products and also used to obtain highly reflective shining surfaces for optical appliances. The electrodeposition process is used to create a durable rhodium coating with a presentable color on jewelry.
Rhodium is also used to produce palladium and platinum alloys that have high hardness and excellent corrosion resistance. These alloys are then used to manufacture catalytic converters and catalytic nets that catalyze chemical reactions. In 1976, three-way catalytic converters were developed by Volvo by using rhodium alloys. This breakthrough helped minimize nitrogen oxide (NOx) emissions from automobiles.
Rhodium is a noble metal, known for its unique corrosion resistance, high temperature chemical stability, durability, shiny appearance and reflectance. It is one of the six rare metals of the platinum group. Rhodium does not tarnish and is not prone to corrosion. It is one of the rarest noble metals with very good durability.
Most of this rare metal is used by the automobile industry to make vehicle catalytic converters that accelerate and catalyze the reduction of nitrogen oxides into nitrogen gas, thus enabling regulatory engine exhaust compliance.

Matharu AS, Wu Y (2008) Liquid crystal displays: from devices to recycling. In: Hester RE, Harrison RM (eds) Electronic waste management, issues in environmental science and technology. RSC Publishing, Cambridge UK
Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on waste electrical and electronic equipment (WEEE) – Joint declaration of the European Parliament, the Council and the Commission relating to Article 9; http://ec.europa.eu/environment/waste/weee/index_en.htm. Accessed 6 Feb 2011
Yuan Z, Shi L (2009) Improving enterprise competitive advantage with industrial symbiosis: case study of a smeltery in China. J Cleaner Prod 17:1295–1302
Eugster M, Huabo D, Jinhui L, Perera PJ, Yang W (2008) Sustainable electronics and electrical equipment for China and the world – a commodity chain sustainability analysis of key Chinese EEE product chains. International Institute for Sustainable Development, pp 1–91
Schluepa M, Hageluekenb C, Kuehrc R, Magalinic F, Maurerc C, Meskersb C, Muellera E, Wang F (2009) Sustainable innovation and technology transfer industrial sector studies: recycling from E-waste to resources. United Nations Environment Programme and United Nations University, Nairobi, Kenya
E-Waste Volume 1 Inventory Assessment Manual (2007) United nations environmental programme division of technology. Industry and Economics International Environmental Technology Centre, Osaka/Shiga
Ongondo FO, Williams ID, Cherrett TJ (2010) How are WEEE doing? A global review of the management of electrical and electronic wastes. doi: 10.1016/j.wasman.2010.10.023
Waste and Climate Change. Global Trends and Strategy Framework (2010) United Nations Environmental Programme, Division of Technology, Industry and Economics International Environmental Technology Centre, Osaka/Shiga, pp 1–79
Boggio B, Wheelock C (2009) Executive summary: electronics recycling and E-Waste issues recycling and responsible disposal of consumer electronics, computer equipment, mobile phones, and other E-Waste. Pike Research LLC, Boulder, USA
Schluep M, Rochat D, Munyua AW, Laissaoui SE, Wone S, Kane C, Hieronymi K (2008) Assessing the e-waste situation in Africa. Electronics Goes Green 2008+, Berlin, Germany, pp 1–6
Parthasarathy P, Bulbule KA, Anantha Murthy KS (2008) E-Waste recycling – best option for resource recovery and sustainable environment. Res J Chem Environ 12(1):93–98
Steubing B, Böni H, Schluep M, Silva U, Ludwig C (2010) Assessing computer waste generation in Chile using material flow analysis. Waste Manage 30:473–482
Ciocoiu N, Burcea S, Tartiu V (2010) The WEEE management system in Romania. Dimension, srengths and weaknesses. Theor Empirical Res Urban Manage 6(15):5–22
Chancerel P, Meskers CEM, Hagelűken C, Rotter VS (2009) Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. J Ind Ecol 13(5):791–810
Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment; http://ec.europa.eu/environment/waste/weee/index_en.htm. Accessed 6 Feb 2011
Basel Convention on the Control of Transboundary Movements of HazardousWastes and Their Disposal; http://www.basel.int/text/con-e-rev.pdf. Accessed 6 Feb 2011
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC; http://ec.europa.eu/environment/chemicals/reach/reach_intro.htm. Accessed 6 Feb 2011
Chang TC, You SJ, Chen CM, Lee YF (2010) Mercury recovery from cold cathode fluorescent lamps using thermal desorption technology. Waste Manage Res 28:455–460
Martin R, Simon-Hettich B, Becker W (2004) New EU Legislation (WEEE) Compliant Recovery Processes for LCDs. IDW 04 Proc of the 11th IDW: 583-586. http://www.lcdtvassociation.org/images/Proceeding_New_EU_Legislation_WEEE_Compliant_Recovery_Processes_for_LCDs-Merck_September_2008n.pdf. Accessed 6 Feb 2011
Lo S-F (2010) Global warming action of Taiwan’s semiconductor/TFT-LCD industries: how does voluntary agreement work in the IT industry? Technol Soc 32(3):249–254
Lei C-N, Whang L-M, Chen P-C (2010) Biological treatment of thin-film transistor liquid crystal display (TFT-LCD) wastewater using aerobic and anoxic/oxic sequencing batch reactors. Chemosphere 81:57–64
You S-H, Tsai Y-T (2010) Using intermittent ozonation to remove fouling of ultrafiltration membrane in effluent recovery during TFT-LCD manufacturing. J Taiwan Inst Chem Eng 41:98–104
Kim Y-J, Qureshi TI (2006) Recycling of calcium fluoride sludge as additive in the solidification-stabilization of fly ash. J Environ Eng Sci 5(5):377–381
Lin K-L, Chang W-K, Chang T-C, Lee C-W, Lin C-H (2009) Recycling thin film transistor liquid crystal display (TFT-LCD) waste glass produced as glass-ceramics. J Cleaner Prod 17:1499–1503
Wang HY (2011) The effect of the proportion of thin film transistor-liquid crystal display (TFT-LCD) optical waste glass as a partial substitute for cement in cement mortar. Constr Build Mater 25:791–797
Nakamichi M Jpn. Kokai. Tokkyo Koho JP 2005 227,508 (Cl. GO2F1/13), 25 Aug 2005, Appl. 2004/35,597, 12 Feb 2004. Decomposition method and apparatus for liquid crystals in recycling of liquid crystal panels. CAN: 143: 219606g
Felix J, Letcher W, Tunell H, Ranerup K, Retegan T, Lundholm G (2010) Recycling and re-Use of LCD components and materials. SID Symp Dig Tech Pap 41(1):1469–1472

We don’t usually tell the histories of precious metals on this blog. But we’ll make an exception in the case of rhodium because the story is so doggone interesting.
Way back in 1803, a British scientist named William Hyde Wollaston used a chemical process to extract rhodium from ores that also contained platinum and palladium. Because the metal is reddish in color, it was named rhodium from the Greek word rhodon, which means “red.”
For a long time, the metal wasn’t widely used, but about 100 years after its discovery, it found its way into laboratory devices that measured high temperatures. Rhodium’s “big break” came in the mid-1970s, when rhodium-containing catalytic converters were first used to reduce the pollutants in automobile exhaust emissions. Since then, the demand for rhodium – both new and recycled – has remained strong.
Rhodium is one of the rarest elements. It is estimated to make up only 0.0002 parts per million of the earth’s crust. The largest known concentrations of it are in the Ural Mountains in Russia, in South Africa, and in Ontario, Canada. Because rhodium is both scarce and expensive to extract from ores, it’s worth a lot and because it is finding increasing use in aerospace, labs, and other applications, its value is almost certain to increase.
The “common knowledge” about rhodium is that it is most commonly found in automobile catalytic converters. But the reality is that not all cat converters contain rhodium – and those that do contain only a tiny amount. Here are some alternative rhodium-containing items that can return higher value to you…Platinum-rhodium alloy mesh, wire, sheet, rods, foil, and tubes that you can reclaim from several industries that include aerospace manufacturing.
Rhodium and rhodium-plated rings, watches, and other items of jewelry. They contain only a tiny amount of rhodium, so you will have to collect a very large number of them to profit from recycling them.
If you do some searching online, you will discover that some items of rhodium-plated jewelry are selling for amazingly low prices – on the order of $30.00 - $100.00. How can that be? It’s because extremely thin layers of rhodium are used as plating. A tiny amount of rhodium is enough to add a bright, shiny surface to a ring or another piece of jewelry.
It is used because it is bright, tarnish-resistant, and abrasion-resistant. It has a beautiful bright finish, not unlike platinum. And it retains all those properties, even when only a thin layer is applied to other metals. If a plating of rhodium is added to silver, for example, that silver becomes more scratch-resistant.
Rhodium can be plated onto gold, silver, platinum, and base metals too. But most of the better quality rhodium-plated jewelry you will find is made of silver that has been given a thin plating of rhodium.
Generally, no. Even though rhodium conducts electricity well, it is no better at doing so than copper, which is far cheaper. So rhodium has not become widely used in the manufacturing of printed circuit boards and other electronic components.
We are called Specialty Metals Smelters and Refiners because we perform highly specialized processes to recover precious metals. And recording rhodium is one of our specialties.
If you have rhodium to reclaim from the kinds of scrap we describe in today’s post, give us a call at 800-426-2344. Our specialists are here to help you profit from all precious metals, including rhodium.

Rhodium is a precious metal that belongs to the platinum group. It is a very rare and expensive element that is extracted from native platinum. A well-known property of the metal is its ability to break down certain substances into their simplest components in a gaseous state. It is known for its high chemical stability to aggressive environments: in this respect it surpasses many other elements.
Wollaston dissolved raw platinum in aqua regia and neutralized the excess acid with caustic soda. From the neutralized solution he covered platinum with ammonium chloride and palladium with mercury cyanide. The filtrate was treated with hydrochloric acid to remove the excess mercury cyanide and evaporated to dryness. The residue treated with alcohol turned out to be a dark red rhodium-sodium salt powder. The rhodium was recovered by heating this powder in a stream of hydrogen.
The largest reserves of rhodium there are today, Mexico (43%) is the frontrunner in terms of gold deposits, as its gold sands are oversaturated with rhodium. This is followed by the South African, Colombian and US supplies.
South Africa is the largest exporter of rhodium (80% by volume). The second largest production country is Russia (11,9%), followed by Canada and Zimbabwe.
The extraction of rhodium from the obtained macro shafts is carried out in an artisanal way (mining). It is complicated by the composition of the ore: the metal is associated with gold, palladium, platinum and copper.
The next step is metallurgy. The concentrated mixture is immersed in an oven and heated to 1500 ° C. The old rock is burned and the slag is removed by blowing it out. The concentrate content rises to 1400 g / ton. -
A solution left over from the platinum refining process is used to release the rhodium. After treatment with chemicals and incineration, the result is almost pure rhodium metal.
Research is currently being carried out on several technologies in order, among other things, to recover rhodium from hazardous waste residues from nuclear power plants. These very cost-intensive new technologies enable a greater production potential of rhodium in the future than from conventional mining. Furthermore, problematic storage of nuclear waste would be reduced in this way.
Iridium or platinum alloys are contained in precision instruments that can measure temperatures of up to 2200 ° C (for example in catalytic converters).
A platinum / rhodium alloy reduces the formation of nitric acid. Such an alloy is used to manufacture exhaust gas neutralizers for motor vehicles. The automotive industry is the main user of rhodium.
They are used to coat mirror surfaces and ultra-precision instruments. This is thanks to the fact that the surface reflects 80% of the light in the visible range of the spectrum and is not clouded by Superfine.
In radio technology, it is mostly not used for the manufacture of components because it is too brittle and unstable. Radio components are simply coated with rhodium. The contacts (reed relays) of the RPS 55 (A), for example, are coated with rhodium.
Electronic engineer of computer technology. Maintenance computer cpu hardware upgrade of motherboard component. Pc repair, technician and industry support concept.
This electronics is developing into an important area of application. The metal is used in filters on LCD monitors (TVs, laptops, iPhones and other devices).
Silver band for engagement with gem for female Cropped of diamond luxury jewelery bijouterie ring from white gold or platinum with gemstone. 3D rendering on black background New collection of jewelry
It has been proven that rhodium compounds are highly toxic carcinogens. However, the amount of rhodium in certified jewelry is harmless to human health.
An alloy of rhodium and copper (or zinc), which is dissolved in hydrochloric acid, filtered and dried, results in a powder. This is because it is a compound that can detonate under normal conditions.
The global rush reached its peak at the end of 2008: At that time, up to 10.1k dollars per troy ounce were in demand on the foreign exchange market, which was ten times more than gold at the same time.
In the last period (December 2019 - December 2020) the price per gram has tripled: from $ 150 to $ 500. In other words, the price was around $ 2021 for an ounce in December 14.100. The all-time high of rhodium was recorded in May 2021 at USD 29.100 per troy ounce.
As with other precious metals, the price of rhodium fluctuates. In the long term, however, it will continue to grow. Science, manufacturing and technology will not do without this metal. However, production capacities are limited and global deposits are finite.

Electronics metal finishing can be a challenging process that requires a finely honed expertise. With 90 years of metal finishing experience at our disposal, you can trust Sharretts Plating Company to provide the best metal finishing process for your electronics industry applications. Few electronics plating companies can match our mastery of plating on semiconductors, connectors, and other vital electronic parts and components. Learn more about our extensive capabilities when plating electronics with various metals.
It"s hard to imagine living in a world without electronic products, gadgets or devices. Products such as computers, cellphones and televisions play a primary role in just about everyone’s lives. We use them at home, at work and even in our cars. Electronic systems now control the operation of automobiles, airplanes and other modes of transportation. Innovation in the electronics industry continues at a breakneck pace, and hardly a day goes by without the unveiling of a new product. It"s safe to say that without electronics, the world would be a much different place.
Although electronic products have been with us throughout our lives, their development has been a relatively recent phenomenon. The electronics industry did not emerge until the early 20th century following the invention of electricity in the late 19th century. Some of the earliest electronic product innovations were the gramophone — a forerunner of the record player — the telephone and the radio. It wasn"t until the 1950s that televisions became a staple in most American households. Personal computers did not gain widespread acceptance until the 1980s.
How important is the electronics industry to the United States economy? According to the Consumer Technology Association, revenues from U.S. shipments of consumer electronics products are expected to reach $222.7 billion in 2015 and $228.8 billion in 2016. This represents the continuation of an upward trend: 2011 revenues were $197.1 billion followed by $206.1 billion in 2012, $210.7 billion in 2013 and $217.6 billion in 2014.
One of the most important processes in the manufacturing of electronic parts and components is electroplating, which involves the application of a metal coating via electrodeposition. This is done for a number of reasons such as improving corrosion resistance, enhancing electrical conductivity, increasing the solderability of the substrate and protecting against wear. Plating electronics can be a difficult process due to the delicate nature of many electronic components. Sharretts Plating Co. has developed expertise in the following area of metal finishing for electronics.
Gold is a precious metal that offers significant value in plating for electronics. When you think of gold, the first thing that probably comes to mind is its glittery appearance. However, gold is also commonly used in the plating of many different electronic components. Although relatively expensive, gold offers low and stable contact resistance and superior protection against corrosion. Gold plating is commonly used in the manufacturing of connectors, contacts, circuits and semiconductors.
Nickel is often used to provide an underlying coating when using gold to plate electronics. Nickel acts as an extra corrosion inhibitor by preventing rust from penetrating pores in the surface. Nickel also prevents the diffusion of other metals into the gold surface such as zinc or copper. What"s more, nickel can increase the durability of the gold-plated surface.
The thickness of the coating is an important consideration when plating on electronics. As a general rule, the coating should be as thin as is absolutely necessary for the application. For instance, a 0.8 micron of hard gold over a 1.3 micron nickel coating will provide sufficient durability for the majority of connector manufacturing applications.
Like gold, silver is a precious metal that offers numerous electronics manufacturing benefits. For one, silver is typically a less expensive alternative for metal finishing electronics. Silver also provides excellent electrical and thermal conductivity. Because of its low contact resistance, silver plating for electronics is often used to provide a coating on highly active copper parts.
Silver plating is also used in connector applications for contact finishes involving higher current power transmission and lower current separable power, and as a connector finish for higher normal force/lower durability signal applications. Silver also possesses strong solderability characteristics. One potential drawback when plating with silver is its tendency to tarnish. Immersion silver is sometimes used for applications requiring solderability, although the shelf life of immersion silver is somewhat limited.
The appropriate thickness of the silver coating is determined by factors such as the harshness of the environment, degree of durability and whether any surface treatments are applied. A thinner silver coating is required if a nickel undercoating is applied. A nickel undercoat can help to limit tarnish and prevent the formation of potentially harmful silver-copper intermetallics.
Another precious metal used for electroplating is platinum. A member of the platinum group of elements — which also includes rhodium, palladium, osmium, iridium and ruthenium — platinum metal is relatively rare, adding to its high monetary value. Platinum metal is silvery-white in color and is known for its attractive, lustrous appearance. Platinum is more ductile than its gold and silver precious metal counterparts, and it"s also extremely malleable. Platinum also offers excellent resistance against corrosion.
In terms of electronics metal finishing, the platinum electroplating process is primarily used to apply a protective coating on low-voltage and low-energy contacts. In addition to preventing the formation of corrosion, a platinum electroplating solution can facilitate electrical conductivity. A platinum coating can range from 0.5 to 5 microns, although an electronics platinum electroplating process is typically designed to produce a coating toward the thicker end of the scale.
Like platinum, rhodium is silvery-white precious metal, although it is much whiter than platinum. Along with the other platinum group metals, rhodium can be found in platinum or nickel ores. Rhodium is also very hard and extremely durable — it will not form an oxide even under extreme temperatures. In fact, rhodium"s melting point is higher than that of platinum. Another valuable rhodium property is its ability to ward off attacks from acid solutions.
A rhodium electroplating process is often used in electronics manufacturing due to rhodium"s low electrical resistance. A rhodium electroplating solution will provide a protective coating on sliding electrical contacts as a means of reducing wear and tear. When applied to high-voltage or high-amperage electrical contacts, a rhodium plating solution will inhibit the formation of oxidation on the contact surface.
Our electronics metal finishing services also include plating with palladium and alloys such as palladium-nickel and palladium-cobalt. Palladium is a somewhat rare, silvery-white metal that is a member of the platinum group. Palladium is frequently used in the manufacturing of connector plates in many types of consumer electronics products. A palladium-nickel alloy can offer the important advantage of low surface contact resistance. The emergence of a functional palladium-cobalt alloy in recent years has proved invaluable in the mass production of electronic components.
Palladium and palladium alloys are gaining acceptance as a more cost-effective alternative to gold in the plating of connectors used to link internal computer components. Because palladium is less dense than gold, palladium plating results in a lower weight of the finished product, while still providing a comparable coating thickness. Some manufacturers are also turning to palladium to coat the lead frames that connect integrated circuits to other electronic devices. Palladium offers a more environmentally friendly alternative to a tin-lead alloy when performing this function.
Most of us know that copper conducts electricity extremely well. It is a relatively soft metal that offers high thermal conductivity. Using copper for plating is also much less expensive than when plating with precious metals such as gold and silver. It"s these properties that make copper highly valuable in plating for electronics parts and components. You"ll find copper plating used widely in the production of semiconductors and circuits.
Copper can also be used as an underplate for other metals in the electroplating process. A coating of copper will enhance the electrical properties of the other metals and increase the corrosion resistance of the deposit. Copper will also increase the throwing power and provide greater deposit consistency.
Tin plating is a relatively low-cost process that is favored by budget-conscious companies for many types of electronics industry manufacturing applications. One disadvantage of “tinning,” as it is widely known, is the formation of tiny metal protrusions called whiskers that can cause electrical shorts. SPC has developed a highly effective tin-lead alloy that can limit the occurrence of whiskers.
Using a tin-lead alloy in plating for electronics also offers a number of additional benefits besides preventing tin whiskers. Tin-lead provides excellent solderability, which is essential in so many electrical and electronics manufacturing applications. Tin-lead typically does not require an undercoating, which simplifies the plating process and minimizes costs. General thickness recommendations when plating on electronics with a tin-lead alloy ranges from 0.0003 to 0.0005 inches.
While plating on plastics is primarily used in the automotive industry to metallize nonmetallic vehicle parts, many parts and components used in the electronics manufacturing industry are now comprised of plastic or other nonconductive material. Using plating to apply a coating of metal such as copper is a way to metallize these parts, which enables them to conduct electricity. Copper is an obvious choice for plating onto plastic electronic parts due to its excellent electrical conductivity and superior thermal properties. SPC is one of the few electronics plating companies in the world that has been able to master this extremely complex and challenging process.
Nickel and nickel alloys are often used to plate on plastic items such as knobs and control buttons on personal computers and electric shavers. A shiny nickel coating can improve the appearance of the product and limit the impact of wear caused by frequent handling. It can also provide a decorative touch to the plastic trim on household appliances such as refrigerators and freezers. The development of more heat-resistant plastics has also led to the use of nickel plating for connector blocks, which facilitates direct soldering onto the surface. Nickel plating and plastics technology can also be combined to develop circuit systems with interconnecting paths.
Examples of the electrical properties exhibited by ceramic materials include:Semiconductor: Certain types of ceramic materials act as semiconductors that conduct electricity under some conditions. This makes them valuable for surge protection applications, particularly in electrical substations to protect against lightning strikes.
Piezoelectricity: Numerous ceramic materials feature a property called piezoelectricity, which serves as a link between a mechanical and an electrical response. One example is the quartz that watch manufacturers use to measure time in watches and other electronic devices.
Additionally, many ceramics possess excellent thermal properties that enable the part or component to resist the extreme temperatures that are common in electronics applications.
The metal coating of electronics can also be achieved without the use of electricity. A process known as electroless plating enables the coating of an electronic part or component via chemical reaction. This results in a more even, uniform coating that is able to coat areas on the substrate that are unreachable with typical electroplating methods.
A primary use of electroless nickel plating in electronics is in the manufacturing of semiconductors. The nickel coating makes the surface more receptive to soldering, brazing and welding than would be possible when plating with an electrical current. Nickel also provides excellent corrosion protection. Electroless nickel plating is also used in the production of zinc or copper heat sinks that keep the semiconductors cool during operation.

Rhodium is a rare platinum group metal (PGM) that is chemically stable at high temperatures, resistant to corrosion and mainly used in the production of automobile catalytic converters.
Rhodium is a hard, silver-colored metal that is very stable and has a high melting point. Rhodium metal is resistant to corrosion and, as a PGM, it shares the group’s exceptional catalytic properties.
In 1803, William Hyde Wollaston was able to isolate palladium from other PGMs and, consequently, in 1804, he isolated rhodium from the reaction products.
Wollaston dissolved platinum ore in aqua regia(a mixture of nitric and hydrochloric acids) before adding ammonium chloride and iron to obtain palladium. He then found that rhodium could be drawn from the chloride salts that remained.
Wollaston applied aqua regia then a reduction process with hydrogen gas to obtain rhodium metal. The remaining metal showed a pink hue and was named after the Greek word "rodon", meaning "rose".
Rhodium is extracted as a byproduct of platinum and nickel mining. Owing to its rarity and the complex and expensive process required to isolate the metal, there are very few naturally occurring ore bodies that provide economical sources of rhodium.
Like most PGMs, rhodium production is focused around the Bushveld complex in South Africa. The country accounts for over 80 percent of the world"s rhodium production, while other sources include the Sudbury basin in Canada and the Norilsk Complex in Russia.
The first step in extracting rhodium from the ore is precipitating precious metals such as gold, silver, palladium, and platinum. The remaining ore is treated with sodium bisulfate NaHSO4 and melted, resulting in rhodium (III) sulfate, Rh2(SO4)3.
Rhodium hydroxide is then precipitated out using sodium hydroxide, while hydrochloric acid is added to produce H3RhCl6. This compound is treated with ammonium chloride and sodium nitrite to form a precipitate of rhodium.
The precipitate is dissolved in hydrochloric acid, and the solution is heated until residual contaminants are burnt off, leaving behind the pure rhodium metal.
According to Impala Platinum, global production of rhodium is limited to only about 1 million troy ounces annually (or roughly 28 metric tons) annually, whereas, in comparison, 207 metric tons of palladium were produced in 2011.
About one-quarter of rhodium production comes from secondary sources, mainly recycled catalytic converters, while the remainder is extracted from ore.Large rhodium producers include Anglo Platinum, Norilsk Nickel, and Impala Platinum.
According to the US Geological Survey, autocatalysts accounted for 77 percent of all rhodium demand in 2010. Three-way catalytic converters for gasoline engines use rhodium to catalyze the reduction of nitrogen oxide to nitrogen.
Roughly 5 percent to 7 percent of global rhodium consumption is used by the chemical sector. Rhodium and platinum-rhodium catalysts are used in the production of oxo-alcohol manufacturing as well as to produce nitric oxide, a raw material for fertilizers, explosives, and nitric acid.
Glass production accounts for a further 3 percent to 6 percent of rhodium consumption each year. Because of their high melting points, strength and resistance to corrosion, rhodium, and platinum can be alloyed to form vessels that hold and shape molten glass. Also of importance is that alloys containing rhodium do not react with, or oxidize, the glass at high temperatures. Other rhodium uses in glass production include:
In the production of liquid crystal displays (LCDs) because of the higher temperatures required to melt raw materials and the quality of glass required.

Glass is manufactured by the melting of specific minerals including silicates and potash to temperatures that often reach and exceed 1700 degrees Celsius. In order to craft containers and molds that can not only withstand these temperatures, but also hold up under the abrasiveness of molten glass, platinum and alloys of rhodium and platinum are used. The high melting point and resistance to corrosion make platinum the most likely material for use under these conditions. Platinum and the various alloys, unlike most base metals, do not react with the molten glass, resulting in a much more pure glass mixture than has been previously available with molds and equipment made from other metals.
The platinum used does not oxidize at these extremely high temperatures and will not scale. By adding from 5% to 30% rhodium as an alloy it is possible to make the platinum even stronger and more wear resilient, which in turn extends the life of all of the equipment significantly. Manufacturing the glass component of the Liquid Crystal Display is said to be the most extensive use of both platinum and rhodium in the industry. There are two reasons for this, first the quality of the glass that is required to make computer monitors and digital watches and secondly that the glass is heated to a minimum of 1650 degrees Celsius. This glass is typically no more than 0.5 mm thick and must be absolutely flawless in order to be used for this particular application.
When manufacturing optical quality glass you will find that platinum plays a very large roll in all phases of manufacture including melting, conditioning and forming the glass. The platinum used for this purpose must be pure as the use of alloys such as rhodium and iridium has been found to cause discoloration of the glass, which is completely unacceptable in this type of application. With the high demand for the purest quality glass in applications such as electronics and the medical field, the demand for platinum has skyrocketed. No longer a simple resource for jewelry or electronics this precious metal is now considered to be one of the better investments as the sources of the raw materials are somewhat limited.

In nature it is found with other platinum group minerals and metals. These characteristics combined with its low electrical resistance makes rhodium commonly used as an electrical contact material for electrical contacts, semiconductor wafers, printed circuit boards (PCBs), and other mission critical components.
RHODIUM. While the major use of rhodium (Rh) is in catalytic converters, 11% of production is used in glass-related applications, such as coatings for optic fibres and optical mirrors. Because it is also highly resistant to corrosion, it is used for thermocouple elements and crucibles.
Platinum, palladium, rhodium and iridium are used to coat electrodes, the tiny components in all electronic products which help to control the flow of electricity.
Rhodium is distinguished by its unique corrosion resistance, hardness, silvery-white metallic appearance and chemical inertness. It does not tarnish and is not prone to corrosion at normal room temperature.
South Africa produces over 85% of the global rhodium supply annually, with majority of this supply being generated by the mining companies listed below (rhodium production listed as a percentage of overall mining production):
Q: What cars have the most rhodium? If you"re asking, “Which catalytic converters have the most rhodium?” Some of the cars with the most rhodium in their cat converters include the Ferarri F430, Ford Mustang, Ram 2500, Ford F250, etc. This is part of the reasons why these cars are luxury automobiles in the industry.
Selling Rhodium Online. At Express Gold Cash we understand that selling your precious metals can be both sensitive and confusing. We work to make sure our customers are 100% satisfied. We accept all forms of scrap rhodium including rhodium bars, rhodium sponges, rhodium alloy wire, sheet, rods, foil, tube, mesh.
The major use of rhodium is in catalytic converters for cars (80%). It reduces nitrogen oxides in exhaust gases. Rhodium is also used as catalysts in the chemical industry, for making nitric acid, acetic acid and hydrogenation reactions.
Computer CPU"s (processors) have the most precious metal value by weight, followed by Memory (RAM) & Circuit Board Fingers / Connectors / Pins, then Circuit Boards (Motherboards), then cables / wires, with hard drives & whole computers being last.
Rhodium is used as an alloying agent for hardening and improving the corrosion resistance of platinum and palladium. These alloys are used in furnace windings, bushings for glass fiber production, thermocouple elements, electrodes for aircraft spark plugs, and laboratory crucibles.
In the manufacture of TFT-LCD panels (used in TVs, monitors and displays), platinum and rhodium are used to line the channels, melting tanks and stirring cells, not only because they can withstand temperatures up to 1,650ºC, but also because they are inert.
Palladium is often used in cell phone and laptop components, and it"s also found in ceramic capacitors having multiple layers. Due to the metal"s high level of conductivity, manufacturers commonly include it in the connector plates of a variety of electronic products.
Typically, the amount of rhodium in a catalytic converter is anywhere between 1-2 grams, while the amount of platinum ranges anywhere from 3 to 7 grams and the amount of palladium ranges anywhere from 2 to 7 grams.
Historically, Rhodium reached an all time high of 29800 in March of 2021. Rhodium - data, forecasts, historical chart - was last updated on December of 2022.
Rhodium is often used by jewelers as a coating on silver, platinum, and palladium jewelry to make the items more scratch resistant and improve luster and shine. Because of its reflexive properties, rhodium is also used in high quality glass and LCD screen production.
Rhodium is a silvery-white platinum group metal (PGM) resistant to corrosion and highly reflective. It is considered the rarest and most valuable precious metal in the world. About 88% of the global rhodium produced is used for making catalysts that reduce the release of harmful substances from vehicle exhausts.
Rhodium is an ultra-shiny, corrosion resistant metal that had become useful in many industries including the automobile, jewelry, chemical and electrical trades. According to Peterson, it"s rhodium"s scarcity and use that makes it so valuable.
Rhodium is a platinum-group metal. In 2022, the supply of rhodium in South Africa was forecast to stand at around 575,000 ounces, making South Africa the world"s largest rhodium producer.

Rhodium, a chemical element with symbol Rh, is a silvery-white, hard, corrosion-resistant, which can be used as a structuring material for glass manufacturing. It exhibits properties such as high melting point and enhanced corrosion resistance.It is naturally occurring, free metal, rarest, and most valuable precious metals. Alloys made from rhodium are used in manufacturing of LCD glass for flat panel displays and many more products.These alloy compositions are used in low-energy &low-voltage contact, thick &thin film circuits, thermocouples &furnaces, and electrodes. South Africais the largest producer of rhodium.
Owing to the lockdown implemented across various countries, national and international transport have been hampered, which has significantly impacted the supply chain of numerous industries across the globe, thereby increasing the supply–demand gap.
Increasing demand for glass manufacturing, LCD glass panels, and auto-catalyzing is driving the demand for rhodium alloys.Increasing use of rhodium by automotive industry is expected to affect the market growth. Volatility in rhodium prices is one of the major restraining factorsof the market. Rhodium alloy market is still in development stage. As rhodium is one of the rarest metals in the world, supply struggles to catch up to demand. Around more than 50% of all rhodium comes from South African mines, in which distribution is difficult, especially with mining strikes in past years.
North America is at the top position to lead rhodium alloys market in terms of market size. Asia-Pacific, being a growth-oriented country in almost every sector, is growing at a significant rate. Latin America is trying to expand its market size in coming years.
Key benefits of the report:This study presents the analytical depiction of the global rhodium alloys industry along with the current trends and future estimations to determine the imminent investment pockets.
The report presents information related to key drivers, restraints, and opportunities along with detailed analysis of the global rhodium alloysmarket share.
The report provides a detailed global rhodium alloysmarket analysis depending on competitive intensity and how the competition will take shape in coming years.
Key Market Players J and J materials, Inc., Pure tech, Merck KGaA, Rhodium ferro Alloys private ltd., American elements, Reade international corp., Nobills metals, Parekh industries, Anglo American




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey