lcd panel test pricelist

Prices for all TV panel sizes fluctuated and are forecast to fluctuate between 2020 and 2022. The period from March 2020 to July 2021 saw the biggest price increases, when a 65" UHD panel cost between 171 and 288 U.S. dollars. In the fourth quarter of 2021, such prices fell and are expected to drop to an even lower amount by March 2022.Read moreLCD TV panel prices worldwide from January 2020 to March 2022, by size(in U.S. dollars)Characteristic32" HD43" FHD49"/50" UHD55" UHD65" UHD------
DSCC. (January 10, 2022). LCD TV panel prices worldwide from January 2020 to March 2022, by size (in U.S. dollars) [Graph]. In Statista. Retrieved January 21, 2023, from https://www.statista.com/statistics/1288400/lcd-tv-panel-price-by-size/
DSCC. "LCD TV panel prices worldwide from January 2020 to March 2022, by size (in U.S. dollars)." Chart. January 10, 2022. Statista. Accessed January 21, 2023. https://www.statista.com/statistics/1288400/lcd-tv-panel-price-by-size/
DSCC. (2022). LCD TV panel prices worldwide from January 2020 to March 2022, by size (in U.S. dollars). Statista. Statista Inc.. Accessed: January 21, 2023. https://www.statista.com/statistics/1288400/lcd-tv-panel-price-by-size/
DSCC. "Lcd Tv Panel Prices Worldwide from January 2020 to March 2022, by Size (in U.S. Dollars)." Statista, Statista Inc., 10 Jan 2022, https://www.statista.com/statistics/1288400/lcd-tv-panel-price-by-size/
DSCC, LCD TV panel prices worldwide from January 2020 to March 2022, by size (in U.S. dollars) Statista, https://www.statista.com/statistics/1288400/lcd-tv-panel-price-by-size/ (last visited January 21, 2023)
LCD TV panel prices worldwide from January 2020 to March 2022, by size (in U.S. dollars) [Graph], DSCC, January 10, 2022. [Online]. Available: https://www.statista.com/statistics/1288400/lcd-tv-panel-price-by-size/

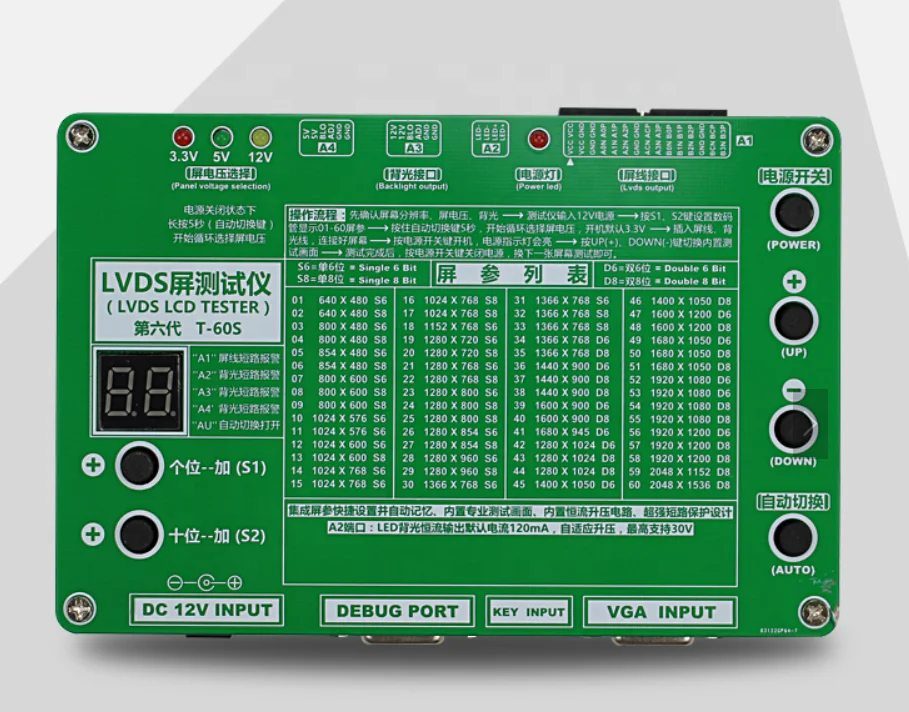
Make screen cable in proper position and prevent driver board from damage with LCD power supply short circuit protection function. The digital number display indicator will show flickering ‘00’ and buzzer will sound if the screen cable is short-circuited. The digital number display indicator will return to normal after removing short circuit. (More than 10 minutes’ short circuit is not allowed).
To use this tool, you need to know the resolution of the LCD/LED screen, screen voltage and the bit number of the LVDS cable for that screen, then check them against the list of screen parameter on the panel to find the right parameter and input it with the “+” key and “-” key on the panel digital.
Then connect the LVDS cable and inverter. Choose the right screen voltage. Press the power key to turn on the power. During the test procedure, if you want to test another screen, you don’t need to unplug the power adapter, just press the power key on the panel to turn the power off.
Two-bit digital tube display area, the corresponding screen reference list through the digital tube below the add and subtract key to display the number of 01-55, LCD screen line power supply short circuit, the digital tube does not stop flashing display “00”, while the buzzer.
The power button (red hat), press the power button, LCD screen and power supply, voltage indicating exhibit of lanterns lit. The power button up the LCD screen, disconnect the power supply. So, in the test, the replacement of the LCD screen just press the power button too.
Screen voltage switch, according to different sizes of LCD screen, to select the appropriate screen power supply voltage, 3.3V/5V/12V optional. Long press the button for 5 seconds, the voltage will automatically jump at the same time, the corresponding voltage indicator lights exhibit of lanterns.
The backlight interface, defined as the standard general high voltage board / 6pin board interface, is used for the external LCD screen of the high-pressure plate/boost plate. Backlight interface, the definition of 6pin is a standard for the general high voltage board/boost board interface for external LCD screen with high-pressure plate/boost plate, for some small size LCD backlight for 5V, can also be used for our future development of various expansion board power supply.
LVDS output port, the foot is defined as the standard definition of the market on the ordinary driver board / common screen line, can be directly bought the screen line to use. Screen switch button for the 12 standard test screen cycle switch.
Automatically switch keys, press a button, the built-in test screen will automatically show, at this time the digital tube two decimal point will be displayed, for the factory assembly, repair and use of the machine.
VGA output, when inserted into the external computer VGA signal, the tester automatically switch to the external VGA signal, do not need to manually switch. Pull back to the built-in test chart display.
LCD tester (Fifth Edition) has done a sound encryption and security protection, unauthorized removal of the test instrument program will automatically remove the lock, and re on the digital tube display 88, along with the ring.

We offer an extensive range of LCD Panels, Moving Display Kit & LED Video Wall. Our never-ending list of LCD panels allows the customers, OEMs & ODMs to enjoy highest level to flexibility when choosing a suitable display for an existing or a new project. However, if you can"t find a appropriate display that is matchingread more...
Advantage: Good price, good quality, Condition: New compatible Product. 100% Compatible & Genuine Product., Super Clarity & Long Life. Premium Quality., Print More & Cost Less. Made with high quality components and superior Materials, produces crisp and deep black text. 100% tested and quality guaranteed; Printread more...
Have you ever properly checked the display quality of the LCD you habitually use? Very often people become aware of previously unnoticed problems in display quality when they run a check using test patterns and so on. This time we are going to talk about the basic points used to assess LCD display quality, and show you a simple way to test it.
Below is the translation from the Japanese of the ITmedia article "The difference in image quality is perfectly obvious! – Let"s check the LCD"s monitor" published April 22, 2010. Copyright 2011 ITmedia Inc. All Rights Reserved.
First of all, bear with us in the following simple test. Below is image data of a row of three squares. In the center of each square is a letter so faint as to be barely distinguishable, so there are three letters in all. Read from the left they make up a word. Can you see that hidden word?
That"s right. The answer is "LCD" (it is displayed if you drag the space between the brackets). We assume that probably many users could read the letters concealed in the squares.
So, the next test is much more difficult. A word is concealed in the four squares below, just as in the image above. The letters are written in colors that are very similar to those of the boxes and we expect that, in many cases, it is hard to distinguish them in your browser. We would like you to download the image and check it closely in photo retouching software or a viewer that is capable of accurate color reproduction.
This time the answer is "EIZO" (it is displayed if you drag the space between the brackets). Depending on the lighting or the user"s environment it may be hard to make out but, if you can read these four letters, the display quality, or more accurately the still image gradation expression, of your LCD is extremely high.
Let"s get down to details then. "Image quality" is the top priority of the LCD, of course. However, recently LCD prices are fiercely competitive and there are surprisingly few products that insist on high image quality and performance. It may be nice to be able to get hold of a wide-screen monitor with full HD (1920 × 1080 dot) resolution or higher fairly cheaply, but it cannot be denied that such LCDs tend not to place too much importance on display quality.
On the other hand, the increasing opportunities to enjoy things like HD videos and games, and high resolution digital photographs on the computer make LCD display quality even more important. As far as possible it"s best to use an LCD with excellent display quality in order to fully enjoy the charms of the visual content.
Even so, perhaps you think that there can"t really be that much wrong with the LCDs that so many people are using at the moment. Here we would like to show you a simple method to check LCD display quality. You can get a good idea of whether the basic display quality is good or bad just by looking at how some simple test images are displayed, just like in the introductory quiz. First of all, we would like you to get a sense of how important it is that "image data can be properly displayed" by checking the display of the LCD that you currently use, (that"s right, the one you are using to view this page!).
The test items use color / monochrome patterned images to check gradation expression, and simple images to check brightness / chromaticity variation. Downloads are available of several test images, such as gradation patterns. We would like you to display the downloaded test images in photo retouching software or a viewer that can reproduce color accurately. As we mentioned at the start of this article, you have to be careful as in many cases colors cannot be displayed accurately in web browsers. (Currently only a few browsers such as Safari and Firefox 3.x can handle color management).
Before starting your visual check of the display quality, please return to your LCD"s setting to default, and select Adobe RGB or sRGB as the image quality mode. If these modes are not available it is fine to set the color temperature to 6500K and gamma to 2.2. If you cannot adjust the color temperature and gamma, simply adjust the brightness and contrast so that they are easier to discern. Of course, if it"s an LCD environment that has been color calibrated it"s OK to leave it as it is.
The average LCD takes some time for the monitor to stabilize after it is switched on so, after start up, please wait at least 30 minutes or so before doing the test. (Most EIZO monitors are an exception to this as they are equipped with our proprietary dimming function and the monitor stabilizes in a short time after start up.)
The surface treatment of an LCD makes a difference to the background reflection. Glare panels impede the surface diffusion of backlight, which does make it easier to achieve high color purity, but also makes distinct reflections of the user or lighting much more likely (photo on the left).
If the lights are similarly trained on a non-glare panel they do not have much effect on the display, only appearing as a fuzzy brightness (photo on the right).
For your reference, we ran a test on an EIZO 24.1-inch wide-screen LCD, the FlexScan SX2462W, for this article. The FlexScan SX series comes with a number of high image quality functions and boasts top class display quality as a general-purpose LCD intended for a computer.
When checking the display quality of an LCD it is comparatively easy to understand the gradation expression capability by a visual check. Let"s display color and monochrome gradation images and check whether the entire image is smoothly reproduced. If there is a problem with the gradation expression it produces things like blocked-up shadows in dark areas and blown-out highlights in light areas, banding (vertical or horizontal stripes) in the middle gradations, and color cast, so you should check for problems like these.
Test images of color / monochrome gradations are shown below. Each test image is prepared for three resolution levels (1280 × 800 dots / 1680 × 1050 dots / 1920 × 1200 dots). When you click on an image it is displayed in that actual resolution. We would like you to download the images in the resolution which matches that of your current LCD. Gradation expression can vary according to whether the image is viewed horizontally or vertically, so it will be more effective if you rotate these images and view them vertically as well.
A gradation pattern where the colors red, green, blue, cyan, magenta and yellow go through 16 gradients as they change to white or black. This is an easy test image so we expect that it can be seen in most environments that each color bar is divided into 16 blocks.
A gradation pattern where the colors red, green, blue, cyan, magenta and yellow go through 64 gradients as they change to white or black. Each color bar is divided into 64 rectangular blocks. With this many gradients we expect that many LCDs will find it hard to make distinctions in the dark areas or the areas that are close to primary colors.
A smooth gradation pattern where the colors red, green, blue, cyan, magenta and yellow go through 256 gradients as they change to white or black. At this level of difficulty you cannot distinguish between adjoining colors from a distance but, if you have an LCD with excellent gradation expression, if you look closely you should be able to see that each color is divided into thin rectangular blocks.
A gradation pattern that changes from black to white. It is divided into 5 horizontal bars: from the top, smooth, 128 gradients, 64 gradients, 32 gradients and 16 gradients. Even if all the differences can be distinguished in the 16 and 32 gradient patterns near the bottom, we expect that there will be some parts in the 64 and 128 gradient patterns where it is hard to see the boundaries between adjoining colors. With this kind of monochrome test image you should also check whether any unnecessary colors are mixed with the gray.
On an average LCD gradations of gray that are close to black tend to appear as blocked-up shadows (gradations of gray that are close to white are displayed comparatively accurately). If your LCD"s OSD menu allows you to adjust the contrast, please try gradually turning down the contrast. Turning down the contrast often makes it possible to see gradations that had been subject to blocked-up shadows or blown-out highlights.
Probably most LCDs will be able to detect some degree of banding and color cast in the middle gradations. Banding in the middle gradations is tone jump (Missing gradations) and, along with color cast, means that the RGB gamma curves are unequal. Unlike blocked-up shadows or blown-out highlights, this is an area that it is hard to improve with adjustments made by the user.
When we looked at these test images on the FlexScan SX2462W, in the smooth gradation there was blocked-up shadows right next to the black but we could distinguish differences in gradations of gray until very close to the black area. When it comes to such subtle gradation distinctions the brightness of the room and the adaptability of the eye come into play, so the range that is visible will vary according to the environment and the individual. The gradation expression was excellent, with almost no blown-out highlights in light areas, middle gradation banding or color cast.
The answer is "The far right" (it is displayed if you drag the space between the brackets). If the other grays looked correct, color may not be being correctly recognized for a variety of reasons, such as the lighting environment or the LCD settings.
Now let"s assess the gradation expression with some slightly different test images. Below are color patterns with a spread of pale colors in gradations close to the dark range and the light range. They are arranged so that a distinction cannot be made between adjoining colors on an LCD with insufficient gradation expression.
We expect that you could roughly get the whole picture in the gradation patterns on the previous page, but in the patterns this time some parts that cannot be seen may have appeared in some cases. As we mentioned earlier, LCDs tend to display gradations close to black as a blocked-up shadows, and color patterns that are close to black are particularly hard to distinguish.
Since there are some parts that cannot be seen, the possibility arises subtle skin colors and tones cannot be accurately recognized when doing things like retouching photographs, though the misrecognition will vary according to the user"s eyesight. People who place importance on color reproduction should probably bear this in mind when they think about replacing their LCD or buying an extra one.
Incidentally, when we checked the FlexScan SX2462W with these tests we could distinguish everything in both the close to white and the close to black patterns. As well as no blown-out highlights or blocked-up shadows, we saw no unnatural color casts.
Every LCD has some degree of brightness and chromaticity variation, but there are many products where the variations become more obvious when the brightness is lowered. A comparison of the brightness and chromaticity variation of a number of LCDs reveals that there is a fairly large difference between products, so this is a point to bear in mind.
If you actually try this test you may be surprised to find more variation than you expected when gray or a near-white pale color is displayed. Generally speaking, the center of an LCD screen is the brightest and it gradually gets darker towards the edges. This is no problem if there is not a big difference in brightness between the central and peripheral areas, but there are some products where this difference is very striking.
Incidentally, this test is also an effective way to test the LCD for dot defects (normal lighting / unlit room). We would like you to check the black display in a darkened environment, for example by switching off all the room lights at night. Although you probably saw the whole screen as uniformly black in a light environment, very often in a dark environment you can find variations in some parts due to light leaks.
The FlexScan SX2462W got good results again when we tried it with the brightness and chromaticity variation tests. The brightness decreased slightly at the edges of the screen, particularly the lower edge, but overall the display was even and pleasing. It is installed with a "digital uniformity equalizer" that measures brightness and chromaticity throughout the screen and makes corrections so that the entire screen is uniform.
Monochrome full-screen displays on a FlexScan SX2462W. Only the screen display is shown. The bottom right is a near-white pale orange. There are not many LCDs that can display this kind of pale color as uniformly as this
However, the pitfall here is that it simply means that "the screen is visible". The thing is that the viewing angle specifications are permitted to use the term "visible" until the display contrast ratio drops to an extremely low 10:1 or 5:1 when the screen is viewed from an angle (the steeper the angle from which the LCD screen is viewed, the more the contrast generally declines). In other words, they do not take into account the display uniformity of the central and peripheral areas of the screen, or the level of chromatic change, when the screen is viewed from an angle.
The ideal viewing angles is that the brightness and chromaticity is very uniform and there is not much chromatic change, even when the screen is viewed from a slight angle. The viewing angles given in the specifications are not really very helpful, but you can judge the standard of the panel type that the LCD (liquid crystal panel) adopts. IPS liquid crystal panels have the least change in brightness or chromaticity when the screen is viewed from an angle, and they are followed by VA panels. An IPS or VA liquid crystal panel can be said to indicate the superior nature of the product itself, so this is often included in the catalog or specifications. It is probably a good idea to look through the catalogs of various products.
On the other hand, monitors installed with cost-effective TN liquid crystal panels are in fact the most numerous. However, the TN type lags far behind the IPS and VA types in terms of characteristic viewing angle changes in brightness and chromaticity. Simply viewing the screen from a slightly different angle makes the coloration change dramatically, and the screen looks completely different according to whether it is viewed vertically or horizontally. If the vertical and horizontal viewing angles in the specifications are different then it is a TN type. There are quite a few products with a 20-inch wide screen or larger where colors look different in the central and peripheral areas even when the screen is viewed straight on.
The display on an IPS panel. Even when viewed from this angle, the displayed content can of course be distinguished completely and the colors also show up really well
The display on a VA panel. Compared with the IPS panel the screen is a little whitish and the chromaticity has slipped, but it is a satisfactory viewing angle for actual use
The display on a TN panel. There is a very clear difference from the IPS and VA panels. The display throughout the entire screen lacks uniformity and there is a yellow cast
The gradation images and monochrome images from earlier in this article can be used as they are to check the viewing angles. Display an image on the whole screen, look at it straight on and check whether the brightness and colors are uniform at the top and bottom of the screen, and in the center and at both sides. Then gradually shift the angle from which you view the screen and check how the brightness and coloration change. If you do this with photographic data as well as the test images, you should be able to get a better sense of the difference in the display.
When we checked the viewing angles of the FlexScan SX2462W there was absolutely nothing to criticize since, in addition to the use of an IPS panel, it is equipped with many high image quality functions, including the afore-mentioned digital uniformity correction circuit. The brightness and chromaticity throughout the whole screen is very uniform, and the coloration hardly changed at all when the viewing angle was changed.
We explained here about easy ways to check LCD monitor quality. How were the results for your current LCD? We think that many people were probably very bothered by the blocked-up shadows and blown-out highlights when the test images to check gradation were displayed, by the middle gradation banding, and by the variations in brightness and chromaticity when the monochrome images were displayed.
As we mentioned at the beginning, recently the number of LCDs with excellent display quality is on the decline. Although we would not go so far as to say that the display quality of inexpensive products is poor. Of course a high quality LCD is indispensable if you want to enjoy using your computer, properly handle the needs of applications that require color reproducibility, and to fully enjoy all the benefits of rich content.
The EIZO FlexScan LCD series has excellent display quality in those regards, and we have no qualms about recommending them to everyone. The product line-up is diverse but each model is clearly ranked according to the purpose to which it is suited and its screen size, and they all guarantee above-standard display quality. They may cost a little more than you had budgeted for but the clear value they offer exceeds their price.
If, after trying these tests, you have doubts about the display quality of the LCD that you usually use, we would certainly urge you to consider an EIZO LCD. We would also recommend that you construct a multi-display environment by making the new LCD your main monitor and the one that you have been using your sub monitor.

The Triplett 8060 CamView Elite Ruggedized Video Test Monitor is a portable battery operated video monitor designed to aid CCTV/Security electronics installers and maintenance technicians. Its high contrast 3.5" LCD screen provides a large image that is especially useful for adjusting camera setup, focus, and positioning, as well as viewing the video signal from DVRs, DVDs,VCRs, or other video equipment. It is supplied with an adjustable wrist band for use as a wrist monitor. A 12VDC (1A) output is provided to power a typical video camera, allowing complete camera setup before the rest of the installation has been completed. BNC video input and output jacks allow video to be "Looped Through" the CamView for local as well as remote display. The rugged yet lightweight construction and ergonomic design maximizes convenience and portability, and the large capacity Lithium Polymer rechargeable battery delivers extended runtime. All video settings (Brightness, Contrast, Color Temp, Sharpness) are adjustable via an On Screen Menu. The Triplett CamView Elite is an efficient time saving test tool that will enhance any professional tool kit.

Replacement equipment that Apple provides as part of the repair or replacement service may contain new or previously used genuine Apple parts that have been tested and pass Apple functional requirements.

Widespread indications for use of molecular diagnostics in various aspects of clinical medicine have driven proliferation of testing. The rapid adoption and continuous technological evolution of molecular diagnostics have often strained the development and maintenance of a functional underlying framework of coding, coverage, and reimbursement policies, thereby presenting challenges to various stakeholders, including molecular professionals, payers, and patients. A multidisciplinary working group convened by the Association for Molecular Pathology Economic Affairs Committee was tasked to describe the complex landscape of molecular pathology economics and highlight opportunities for member engagement. In this article, on the basis of review and synthesis of government regulations and procedures, published payer policy documents, peer-reviewed literature, and expert consensus, the Working Group navigates the ecosystem of molecular pathology economics in terms of stakeholders, coding systems and processes, coverage policy determination, and pricing mechanisms. The composition and interrelatedness of various working groups and committees are emphasized to highlight the functional underpinnings of the system. Molecular professionals must be conversant in the language and complex inner workings of molecular pathology economics to lead successful, viable laboratories and advocate effectively for policy development on their behalf. This overview is provided to be a resource to molecular professionals as they navigate the reimbursement landscape.
The economic landscape of molecular pathology testing can be overwhelming as it is an increasingly complex world of stakeholders, regulations, processes, and acronyms. This overview of molecular pathology economics has helped to prepare new members of the Association for Molecular Pathology (AMP) Economic Affairs Committee for service: it is also intended to serve as an educational resource for residents, molecular pathologists, and clinical molecular geneticists, as well as laboratory directors and hospital/laboratory administrators who seek to understand the complex landscape of molecular pathology economics. This overview starts by defining the major stakeholders and then takes the reader through the areas of coding, coverage, and reimbursement, as applied to molecular pathology diagnostics. An understanding of the complexities and challenges facing the molecular pathology community from an economic perspective is crucial for molecular diagnostic laboratories to be successful, as well as for practitioners to participate in effective advocacy and policy development.
In molecular diagnostic testing, individual providers are a broad group of specialty-trained laboratory medicine professionals, including molecular pathologists (typically board-certified M.D. pathologists), clinical molecular geneticists (typically board-certified Ph.D. laboratorians), and medical technologists, collectively referred to in this primer as molecular professionals. Often, these providers are represented nationally and in policy discussions by professional organizations. The involvement of providers in the molecular pathology economic issues, however, is not limited to professional organizations. Individual molecular professionals engage in economic discussions directly through involvement in a wide spectrum of committees and may provide written or public comments to coverage and pricing determinations of both public and private payers.
Laboratory services accounted for approximately 2% of all Medicare Part B payments in 2016 (Department of Health and Human Services, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Statistics-Reference-Booklet/Downloads/2016_CMS_Stats.pdf, last accessed February 7, 2019). Medicare provides payments for approximately 1300 laboratory tests at a value of approximately $8 billion annually (Department of Health and Human Services, https://oig.hhs.gov/oei/reports/oei-09-16-00100.pdf, last accessed October 13, 2018). As discussed in more detail in Pricing, most laboratory tests are paid under a system called the Clinical Laboratory Fee Schedule (CLFS), whereas those tests that are always interpreted by physicians (eg, biopsies and immunohistochemical studies) are paid under a Physician Fee Schedule (PFS). The impact of Medicare coverage and payment policies extends well beyond the program itself because these policies exert significant influence on the practices of both state Medicaid programs and commercial carriers.
Private insurance covers more lives than does Medicare; however, medical care rates generally increase with age, so the Medicare population accounts for a larger proportion of medical costs. Depending on the type of molecular testing (ie, oncology, germline, or infectious disease), coverage for and payment policies of commercial insurers may vary more or less drastically compared with Medicare. Although inherited disease (germline) testing occurs more often in the non-Medicare population (eg, pediatric or pregnant patients), inherited disorders are also relevant considerations in older patients (eg, those with unexplained heart failure, patients with hereditary breast and ovarian cancer syndrome, or those with renal disease). In contrast, molecular testing in oncology (somatic testing) is most prominent in the Medicare population but by no means limited to that group. Although molecular testing for infectious diseases and human leukocyte antigens is common, and many of the policies and processes discussed in this work apply to them, discussion in this primer has been limited to germline and somatic testing.
Patients are the ultimate beneficiaries of access to molecular pathology services. Patients across the spectrum of medicine can and do benefit from molecular services; however, most patients are unaware of this distinct medical subspecialty. Concert Genetics reported that as of March 2019 the total number of genetic tests on the market was >70,000 and includes single-gene tests, multigene panels, and whole exome sequencing tests (Concert Genetics, http://www.concertgenetics.com/wp-content/uploads/2018/04/12_ConcertGenetics_CurrentLandscapeOfGeneticTesting2018.pdf, last accessed November 20, 2019). Utilization of molecular pathology services varies based on disease type and several other factors, and the proportion of patients who benefit from molecular pathology services continues to grow (Personalized Medicine Coalition, http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The_PM_Report.pdf, last accessed October 24, 2019). Advances in identifying clinically useful genetic markers, diagnostic methods, and related pharmaceutical interventions continue to increase, particularly in the oncology space, and patients are becoming more aware than ever of the value of molecular genetic testing.
Patient advocacy organizations are increasingly recognizing the role of molecular pathology and the need to engage in and respond to reimbursement policies that affect patient access to these procedures. Patient advocates, including individuals or an organization, work to improve the care and life of patients, including patient rights, support, education, and care. Many patient advocacy organizations exist as disease-centric nonprofit organizations (eg, American Cancer Society and American Diabetes Association) and work diligently to monitor both Medicare and private payer policies that may affect patient access to services, including molecular testing. For example, both Facing Our Risk of Cancer Empowered (a hereditary cancer organization) and the Ovarian Cancer Research Alliance have played large roles in engaging with CMS regarding both Medicare and Medicaid coverage policies that would affect patient access to appropriate molecular testing. Facing Our Risk of Cancer Empowered has engaged with CMS regarding Medicaid policies for molecular testing and as lead advocate in responding to recommendation statements on BRCA-related cancer released by the US Preventive Task Force (Facing Our Risk of Cancer Empowered, https://www.facingourrisk.org/advocacy/advocacy-category.php?id=4, last accessed February 20, 2019). In addition, the National Organization for Rare Disorders supports the development and access to diagnostics to speed early diagnosis and supports updates to diagnostic reimbursement to better reflect their importance.
Patient advocacy organizations work independently to promote coverage of molecular testing within their specific disease focus, but also work together to form coalitions to streamline engagement with the larger stakeholder community to advance health care policy, including reimbursement for molecular testing. A great example of these is evident in the work that LUNGevity, the largest national lung cancer–focused nonprofit, is doing. With its Take Aim Initiative, it is working with several aligned and engaged stakeholders to ensure that patients have access to testing to help guide their treatment decision in a timely way (LUNGevity, https://lungevity.org/public-policy/access-to-biomarker-testing, last accessed November 20, 2019). The growing patient voice has become crucial in crafting and responding to reimbursement policy, particularly in discussions of coverage and the standards of evidence needed to justify coverage of medical services.
Several different coding systems that are used in US health care and most relevant to molecular genetic testing are explained in brief in this section. The coding system most immediately applicable to molecular genetic testing is the AMA CPT system, which is a level 1 Healthcare Common Procedure Coding System (HCPCS) code; others include HCPCS level 2 codes, including G codes, code modifiers, and Z codes. Finally, International Classification of Diseases, Tenth Revision (ICD-10) codes are discussed, which categorize diseases and conditions, rather than items or services.
Category II codes are five-digit alphanumeric codes (4 digits followed by letter F) utilized along with the category I codes to track nationally established performance criteria for good patient care. Category II codes facilitate quality data collection; they are not associated with any relative value and are billed with a $0.00 billable charge amount. For instance, code 3155F refers to cytogenetic testing performed on bone marrow at time of diagnosis or prior to initiating treatment (American Medical Association, https://www.ama-assn.org/practice-management/category-ii-codes, last accessed August 21, 2018; and eMDs, http://www.e-mds.com/what-are-cpt-ii-codes-and-how-are-they-used-medical-billing, last accessed August 21, 2018). Performance measures are now moving away from claims-based reporting, making these codes less and less utilized.
AMA CPT developed the CPT Proprietary Laboratory Analysis (PLA) code set to accommodate requirements established by Section 216 of the Protecting Access to Medicare Act (PAMA) and subsequent final rule [45 CFR § 162.1002 (2014)]. The CPT PLA code set allows laboratories or manufacturers to specifically identify and track their test. New PLA codes are reviewed and voted on quarterly by the AMA PLA Technical Advisory Group and then subsequently are reviewed by the CPT Editorial Panel. Following approval by the panel, PLA codes become effective the quarter immediately following their publication online by AMA. In addition, the CPT Editorial Panel approved in 2019 and made effective January 1, 2020, the addition of a new symbol (↑↓) to indicate a PLA code that has been approved for category I status. This symbol also indicates that although this code has achieved category I status, the code will remain in the PLA code section of the CPT code book (American Medical Association, https://www.ama-assn.org/system/files/2019-08/may-2019-summary-panel-actions.pdf, last accessed April 1, 2020).
The requirements for PLA codes are less stringent than those for category I/III codes: the test must be performed on human specimens and requested by the clinical laboratory or manufacturer. Each PLA code has a CPT descriptor, and a subset of PLA codes are advanced diagnostic laboratory tests (ADLTs), a special category of tests subject to different policy and payment generated under PAMA, which was passed into law in 2014 and is explained in more detail below. An ADLT is defined as a test (ie, offered and furnished only by a single laboratory) that meets one of the following criteria: the test is an analysis of multiple biomarkers of DNA, RNA, or proteins that, when combined with a unique algorithm, yields a single patient-specific result, or it is a sole-source test cleared or approved by the US Food and Drug Administration (FDA; American Medical Association, https://www.ama-assn.org/practice-management/cpt/cpt-pla-codes, last accessed March 20, 2020). Applications for ADLT status are a separate process from PLA code obtainment through AMA and are processed through CMS on a quarterly basis (Centers for Medicaid & Medicare Services, https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Advanced-Diagnostic-Laboratory-Tests.html, last accessed January 3, 2019).
The 2020 Medicare payment for the G0452 code is $19.13 (may be higher or lower in some regions of the country) for any molecular test interpretation, regardless of complexity.
Another commonly used modifier, -59, is used to define distinct procedural services that are not normally reported together but are appropriate under certain circumstances. One example in molecular pathology would be the inclusion of CPT codes for fluorescence in situ hybridization and microdissection (done in conjunction with tumor testing) for the same patient on the same date of service. Normally, fluorescence in situ hybridization and microdissection codes billed in combination would be flagged as incompatible (and therefore denied); however, because the fluorescence in situ hybridization analysis and microdissection are for separate assays, the −59 modifier would be appropriate. The definition of CPT modifiers is identified in Appendix A of the CPT manual.2
The Molecular Diagnostic Services (MolDx) Program, generated in 2011, is a process established and administered by the MAC Palmetto GBA to evaluate the analytical and clinical validity as well as clinical utility of individual assays, including laboratory-developed molecular tests, to determine coverage and reimbursement. The program professes to hinge on three components: i) test registration and identification, ii) review of an application for coverage, and iii) determination of coverage (if any) and price (if the service is not already nationally priced by CMS) (Palmetto GBA, https://www.palmettogba.com/palmetto/moldx.nsf/docsCat/MolDx%20Website∼MolDx∼Browse%20By%20Topic∼General, last accessed August 28, 2018). The MolDx Program currently covers multiple jurisdictions, 28 states total, which are otherwise overseen by other MACs, as numerous MACs have adopted it (Figure 1).
In the MolDx model, each individual molecular assay is tracked by a Z code. The Z codes are unique five-character alphanumeric tracking codes used to identify an individual molecular diagnostic laboratory test and allow transparent tracking of relevant utilization and technical information about a test on the shareable Change Healthcare Diagnostics Exchange.3 Laboratories submit a request for a Z code and, in some cases, submit validation documentation and references for a technology assessment, which is reviewed by Palmetto in its coverage determination process. Z codes are submitted on claim forms along with the relevant CPT code for that service. According to the MolDx program, the combination of Z codes and CPT codes is meant to facilitate test identification, facilitate coverage determination, and establish reimbursement (Palmetto GBA, https://www.palmettogba.com/palmetto/MolDX.nsf/vMasterDID/8N3ELL4072?open, last accessed February 16, 2018).
The ICD, of which ICD-10-Clinical Modification is the current edition, is a system of classification that describes the clinical diagnosis, condition, or scenario associated with a specific health care encounter. Appropriate ICD codes may accompany the diagnostic or clinical procedure code (CDC, https://www.cdc.gov/nchs/icd/icd10cm.htm, last accessed May 15, 2017). The ICD is copyrighted and published by the World Health Organization. In the setting of molecular pathology testing reimbursement, ICD codes help providers support the billing of a CPT code by providing information on the clinical context for which the procedure was performed. Payers use ICD codes to determine whether the patient circumstances for the procedure meet coverage criteria.
The CPT Editorial Panel is authorized and charged by the AMA board of trustees to develop, maintain, and revise the CPT (American Medical Association, https://www.ama-assn.org/about/cpt-editorial-panel/cpt-purpose-mission, last accessed March 20, 2020). The CPT Editorial Panel comprises 17 members, 11 of whom are physicians nominated by the national medical specialty societies. In addition, physician representatives from Blue Cross/Blue Shield, America"s Health Insurance Plans, the American Hospital Association, and CMS are included as part of the panel. Finally, two representatives from the Healthcare Professional Advisory Committee round out the group. These professionals include pharmacists, psychologists, physical therapists, and other groups. The CPT Editorial Panel also has an Executive Committee. These five individuals comprise the chair, the co-chair, and three members at-large. At least one Executive Committee member at-large must be from a payer group.
Additional groups advise the CPT Editorial Panel on several matters related to molecular pathology (Figure 2). The CPT Advisory Committee, a group of mainly physicians nominated from the national medical specialty societies who are seated in the AMA House of Delegates, exists to support the work of the CPT Editorial Panel. Nonphysician members drawn from the AMA Health Care Professionals Advisory Committee make up part of the CPT Advisory Committee as well. The primary aim of the committee is to advise the CPT Editorial Panel in developing, approving, and editing the CPT code set by providing expert advice on current medical practice. New codes proposals, as well as requests to modify codes, are circulated to this group for comment. The advisory committee will recommend nomenclature for procedures, provide references from the medical literature in support or denial of a specific procedure, and advise on necessary revisions in the code set relevant to each member"s area of expertise. The CPT Advisory Committee is also involved in the generation and editing of AMA educational materials addressing CPT issues.
Ad hoc workgroups with subspecialty expertise advise the editorial board. There are two advisory groups that are specific to pathology and laboratory medicine, which advise on matters specific to molecular codes as the need arises. The MPAG, which is a standing subcommittee that advises the CPT Editorial Panel on nomenclature and applicability of new and revised codes in the molecular diagnostics category, is tasked with review of all coding change applications (CCAs) that are submitted to the AMA regarding molecular pathology. The MPAG then makes its recommendations to the panel and makes them available to the PCC to assist with its deliberations.
The PCC reviews all laboratory and pathology codes, including molecular codes that have also been reviewed by the MPAG. The PCC, generated by the AMA but supported by the College of American Pathologists" staff, provides formal recommendations to the CPT Editorial Panel. The charge of this committee is to develop a consensus on new and revised codes in pathology and laboratory medicine and bring this recommendation to the CPT Editorial Panel. The PCC was generated because many CPT Editorial Panel members are unfamiliar with the unique aspects of laboratory medicine and the PCC also allows the broader laboratory community (including ACLA, the American Society for Microbiology, Advamed, and other groups) to have a direct voice in the generation of CPT codes relevant to the practice of anatomic and clinical pathology. The chair of the PCC is responsible for presentation and defense of all codes presented to the editorial panel that fall in the purview of pathology and laboratory medicine. The AMP currently occupies a rotating seat on the PCC.
The newest relevant committee is the Proprietary Laboratory Analysis Technical Advisory Group and was formed in response to requirements set out by PAMA and the resultant generation of PLA codes by the AMA. Requests for new PLA codes are submitted quarterly by the laboratory or manufacturer offering the test. The requests are reviewed by the PLA Technical Advisory Group, whose primary responsibility is to ensure that the assay meets claims made by the manufacturer and to edit the descriptor for consistency with CPT format. The PLA Technical Advisory Group then makes recommendations on the new PLA codes to the CPT Editorial Panel, who will ultimately vote to approve the codes. A summary of the various groups in the AMA (as well as CMS and other professional societies) with impact on the economics of molecular pathology is provided in Figure 2.
The AMA CPT Editorial Panel oversees the code change application process for new codes or revisions to existing codes. The initial step in the process is completion of a CCA and submission to the AMA by one of the three annual deadlines. Some of the components required by the AMA, along with the proposed code descriptor for the procedure, include the following: a clinical vignette describing the typical patient; a clear description of the service; the rationale for the proposed CPT code relative to existing CPT codes; reference to the supporting literature; the number of laboratories performing the procedure and the annual test volume; and copies of laboratory protocols and example reports (American Medical Association, https://www.ama-assn.org/practice-management/CPT-coding-change-request-instructions, last accessed August 28, 2018).
The CCA can be completed by any interested party, including an individual physician, physician practice, hospital, payer, or company. The CCA process is administered by AMA CPT Editorial Panel staff. The panel requires all laboratory-related code applications be reviewed by the PCC, which is managed by the College of American Pathologists. The PCC is composed of representatives of pathology and laboratory organizations. In addition, the panel generated the MPAG to review all molecular pathology–related applications and provide recommendations to the PCC. The PCC will then make recommendations for each pathology CCA to the CPT Editorial Panel. The CPT Editorial Panel then meets to discuss and votes whether to accept or reject each application during each CPT Editorial Panel meeting. Final votes are posted to the AMA CPT website about a month after each CPT Editorial Panel meeting. Applicants have the option to participate and answer questions by the MPAG, PCC, and/or CPT Editorial Panel. A summary of a code"s lifecycle is provided in Figure 3
Coding life cycle. Codes are generated or revised through either the coding change application (CCA) process for submission of a new or revised Current Procedural Terminology (CPT) code (blue) or the proprietary laboratory analysis (PLA) application process for submissions of a new or revised CPT PLA code (green). The CPT Editorial Panel takes into account comments and recommendations from the Molecular Pathology Advisory Group and the Pathology Coding Caucus. Once a code is generated, subsequent and separate processes exist for both pricing and coverage of that code. AMA, American Medical Association; CMS, Centers for Medicare & Medicaid Services; LCD, local coverage determination; NCD, national coverage determination.
This section will lay out the history of how CPT codes for molecular pathology procedures have evolved and then describe each group of category I CPT codes for molecular testing, focusing on germline and somatic testing; those groups are molecular pathology procedures (tier 1 and tier 2), multianalyte assays with algorithmic analyses, genomic sequencing procedures, and the molecular pathology unlisted procedure code.
The first appearance of molecular-focused CPT codes was in 1993, with the addition of a molecular diagnostics section to the chemistry laboratory section, which included several separate codes for individual steps of molecular testing. From 1993 to 2002, additional CPT codes were added for other molecular services. These codes were structured differently than today"s molecular codes as codes existed for each step in a molecular procedure (eg, nucleic acid extraction, amplification, and molecular probes). A laboratory performing an individual molecular assay identified each technical step within the assay and billed per the assembled codes. This practice became known as stacking codes because they were used in sets and multiples (eg, DNA extraction × 1 plus probe amplification × 10). By the early 2000s, some flaws in this coding system became evident; chief among them was that payers were only able to discern that a molecular assay had been performed, but the specific analyte could not be identified.
Between 2009 and 2013, intensive efforts were undertaken to overhaul molecular pathology coding. AMP sponsored an effort to develop a new coding scheme for molecular tests and produced a white paper describing one possible approach (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/position-statements/2009/AMPCPTReformProposal_Final.pdf, last accessed September 4, 2018). In 2009, the AMA CPT Editorial Panel generated the Molecular Pathology Working Group and charged it with the development of a new coding scheme. The development of the current CPT codes for molecular pathology services was the result of several years of work from a diverse group of stakeholders, but was based heavily on AMP"s recommended structure. Of key importance to this newly devised coding strategy was a method-agnostic approach, focused on specific genes and/or conditions, which by design was different from the previous method-based approach.
Category I CPT molecular pathology procedure CPT codes are divided into two sections, or tiers: tier 1 and tier 2. Tier 1 codes (81170 to 81355) include commonly performed analyte-specific or other well-described analytic targets (eg, CFTR screening, EGFR mutation testing, and chimerism analysis). Tier 2 includes less commonly evaluated analytes, and they are grouped into nine tier 2 codes. The nine codes in tier 2 (81400 to 81408) correspond to nine levels of increasing technical complexity.
The initial distribution of analytes (typically genes) into tier 1 and tier 2 designations was determined based on surveys of some laboratories to determine tests that had the highest volume. Each year, some analytes are moved from tier 2 to tier 1 on the basis of volume increases and the need for more specific and granular coding. For example, in 2017, gene IDH1 went from classified under tier 2 code 81403 to its own tier 1 code, 81120. These changes are proposed and reviewed through the code change process.
Although placement of an analyte into a tier 2 code is not intended to reflect reduced clinical usefulness or that it is research, some payers viewed the tier 2 category codes in this way. However, the challenge remains that generation of a separate code for all clinical genes and targets used in human clinical testing may not be feasible because of the number of targets.
Analyte-specific CPT molecular codes were first published in January 2012; however, CMS deferred adoption of the new codes until 2013. Around this same time frame, stakeholders recognized the growing utilization of then-new technology, next-generation sequencing (NGS; alias massively parallel sequencing). This technology coincided with and enabled the increasing clinical deployment of testing that targeted multiple genomic regions simultaneously, thus reducing the need for sequential or iterative testing of one gene or target at a time.
To address this new coding challenge, AMP developed a draft coding structure to describe genomic sequencing procedures that was presented at a subsequent AMA stakeholder meeting in 2013 (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/position-statements/2013/AMPProposaltoAddressCodingforGenomicSequencingProcedures_FINAL.pdf, last accessed December 20, 2018). AMA formed disease-related expert workgroups to develop the code descriptors, which led to publication of the first set of genomic sequencing procedure codes on January 1, 2015. These codes are designed to capture panel-based testing across a spectrum of clinical scenarios, generally grouped by clinical indication, as well as larger assays that query a much larger fraction of the genome (eg, exome and genome). These codes are also designed to be method agnostic, and it is not imperative that NGS is utilized if the panel-based descriptor of testing has been satisfied. As in other areas of coding, since the original publication of these genomic sequencing procedure codes, new codes have been proposed and adopted, and some codes have undergone changes [eg, the minimum gene list for hereditary colon cancer disorders (CPT 81436) increased from 7 in 2015 to 10 in 2017].
In molecular testing, the unlisted procedure code 81479 may be used until a new specific code is established (American Medical Association, 2017). This code should only be used when no existing code appropriately describes the service provided, and it cannot be multiplexed. Submitting claims using an unlisted procedure code generally involves inclusion of additional documentation describing the procedure and its medical necessity. Claims without supporting documentation are generally denied. In addition, if an appropriate existing code applies, the claim will be denied.
Procedure-to-procedure edits and medically unlikely edits (MUEs) were developed by CMS to reduce claims error rates and fraud in Part B billing for Medicare claims, and the program was implemented on January 1, 2007 (Centers for Medicare & Medicaid Services, https://www.cms.gov/medicare/coding/nationalcorrectcodinited/mue.html, last accessed August 28, 2018). CMS"s national correct coding program provides computer-driven edits on tens of thousands of services where the edit is considered so evident that it does not require a local coverage determination (LCD) or a national coverage determination (NCD; Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/index.html, last accessed August 28, 2018).
There are two formal processes Medicare uses to develop and disseminate coverage decisions and criteria for utilization of medical services: NCDs and LCDs.4 The main outcome of either process is to define the clinical scenario and parameters for a medical service, which are paired with appropriate ICD-10 codes and CPT codes, to establish the coverage criteria. NCDs and LCDs define the clinical scenario necessary for coverage, as well as limited or noncoverage, of specific clinical procedures. They involve services in benefit categories that fall under Medicare Part A or B (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Downloads/FR09262003.pdf, last accessed August 28, 2018). NCDs are binding across every Medicare geography, whereas LCDs only apply in a specific jurisdiction in which they have been promulgated. If there is contradicting information between an NCD and an LCD, the NCD supersedes the LCD (Noridian, https://med.noridianmedicare.com/web/jea/policies/ncd, last accessed August 28, 2018).
As nearly all CMS coverage decisions for molecular tests are LCDs, understanding the LCD process is crucial for understanding molecular pathology reimbursement and coverage by CMS. In addition, because many private insurers and states promulgate Medicaid base coverage and reimbursement decisions in part on Medicare LCDs, understanding the role of LCDs helps to broadly frame the context for coverage decisions across the board. Chapter 13 of the CMS Program Integrity Manual governs the process for generating and revising LCDs (Centers for Medicare & Medicaid Services, https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/pim83c13.pdf, last accessed August 28, 2018).
LCDs may be generated at the initiative of a MAC or based on a request submitted to the MAC from stakeholders within its jurisdiction. There are five main reasons that a MAC will develop an LCD: the MAC identified an item or service that is not covered under certain circumstances and wishes to establish automated review; frequent denials are issued or anticipated; a contractor has assumed the LCD development workload of another contractor and is undertaking to make LCDs more uniform across jurisdictions; a multistate contractor is undertaking an initiative to generate uniform LCDs across its jurisdiction; and an LCD is needed to ensure beneficiary access to care.
LCD coverage decisions should be based primarily on published authoritative evidence derived from randomized clinical trials or other definitive studies as well as general acceptance by the medical community (standard of practice; Centers for Medicare & Medicaid Services, https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/pim83c13.pdf, last accessed August 28, 2018). In addition, the medical community practice should be based on scientific data or research studies published in peer-reviewed medical journals, consensus of expert medical opinion (ie, recognized authorities in the field), technology assessments, or medical opinion derived from consultations with medical associations or other health care experts.
Ultimately, the decision to publish and implement an LCD rests with the MAC medical director. However, there are two formal processes for input on a proposed LCD. First, every proposed LCD must first be reviewed by a Contractor Advisory Committee (CAC) before finalization. Each state hosts a CAC, and CAC membership draws from physicians, beneficiaries, and other health care providers; relevant medical specialties are typically represented by one member from that specialty. The CAC serves as a formal mechanism for physicians in the state to be informed of and participate in the development of an LCD in an advisory capacity. CAC members often relay input from professional organizations; within pathology, the CAP and AMP are both involved in drafting responses to proposed LCDs relevant to pathology practice. Second, LCDs are posted for public comment for 45 days, allowing any stakeholder (including professional organizations) to submit a public comment. There is a legal process for appealing an LCD to a CMS administrative law judge. Also, individual claims denied under an LCD can be appealed on a one-by-one basis based on unique circumstances of the individual case.
Relevant stakeholders, especially physicians, have voiced several criticisms of the LCD process. The accrual of background information leading to an LCD is often not as systematic and comprehensive as desired. This has led to the perception by some that the decision-making process behind LCDs often lacks a degree of transparency. Also, some CAC members believe that their input into proposed LCDs may not always be deeply considered by their MAC before LCD finalization. In response to these and other criticisms, legislation broadly supported by the laboratory community was introduced in the House and Senate in 2017 (Congress.gov, https://www.congress.gov/bill/115th-congress/senate-bill/794 and https://www.congress.gov/bill/115th-congress/house-bill/3635, last accessed July 19, 2019). The Local Coverage Determination Act of 2017 included several provisions, including open meetings, upfront disclosure, meaningful reconsideration and options for appeal, and discouraging the use of LCDs as a backdoor for national coverage; as many MACs have adopted the MolDx program, LCDs issued by Palmetto and then promulgated to all other MolDx jurisdictions result in a de facto NCD (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/position-statements/2013/MolDx%20Coverage%20Letter%20and%20Attachments%2010302013%20FINAL.pdf, last accessed September 4, 2018). Ultimately, the bill was passed in the House but not the Senate. The future of any LCD legislation is unclear. However, and likely in response to this legislation, CMS announced in October 2018, revisions to Chapter 13 of the Program Integrity Manual, where CMS adopted some of the recommendations contained within The Local Coverage Determination Act of 2017 (Centers for Medicare & Medicaid Services, https://www.cms.gov/newsroom/fact-sheets/summary-significant-changes-medicare-program-integrity-manual-chapter-13-local-coverage, last accessed July 19, 2019).
In contrast to LCDs, NCDs are written, reviewed, and issued by CMS directly rather than through a MAC. Requests are made through a formal request letter with accompanying documentation that includes supporting medical and scientific literature along with other relevant information, such as any clinical trials or studies currently underway with bearing on the request (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Downloads/FR08072013.pdf, last accessed August 28, 2018). The NCD process is reliant on a systematic written review of the relevant literature, technology assessments, and input from various stakeholders. For each NCD, CMS may also commission a formal health technology assessment by the Agency for Healthcare Research and Quality (usually via its academic medical center subcontractors) or review by the Medicare Evidence Development and Coverage Advisory Committee that reviews the evidence for an NCD and makes recommendations to CMS on coverage. NCDs are considered binding positions of the CMS agency. Although its use is rare, there is also a legal appeal process to challenge NCDs that




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey